
Fictional patient. For illustration purposes only.
4CMenB (Bexsero) – the first and only Men B Vaccine now available in the Philippines1-3
The Philippine Food and Drug Authority recently approved Bexsero, GSK’s Meningococcal Group B Vaccine (recombinant, adsorbed), for the prevention of invasive meningococcal disease caused by Neisseria meningitidis serogroup B in individuals aged 2 months and older.1,4
This should be a welcome relief in light of the lingering threat of invasive meningococcal disease. With the introduction of Bexsero in the Philippine market, the circle of protection against the various N. meningitidis serogroups and other local causative agents of bacterial meningitis is complete.5,6 And the tides of the battle, particularly against serogroup B meningococcal disease, may finally be at a turning point.
The World Responds: WHO’s Defeating Meningitis by 2030 Roadmap
In recognition of the alarming threat and burden of meningitis, the World Health Organization developed Defeating Meningitis by 2030, a global roadmap approved by the World Health Assembly in 2020 and endorsed unanimously by WHO member states. It aims, by 2030, to eliminate bacterial meningitis epidemics, including Men B, reduce vaccine-preventable cases by 50% and deaths by 70% and reduce disability after meningitis.7
The Insidious Threat of Men B in the Philippines
Meningococcal disease remains a significant global public health threat. Although rare, its two primary clinical forms, meningitis and meningococcal sepsis, are both acute serious illnesses with devastating complications. The disease sets in insidiously – with symptoms often resembling influenza – making it a diagnostic challenge. This, together with its rapid disease course, results in a high mortality rate with death possible within 24-48 hours of onset in up to 50% of those who don’t receive the right treatment. 20% of survivors are also at risk for developing debilitating sequelae.7-10
In the Philippines, majority of invasive meningococcal disease is caused by the serogroup B of the causative agent, N. meningitidis.7,11 While it can affect people of all ages, children less than 5 months of age are at the highest risk, highlighting the need for, effective protection in this age group.12 Studies have also reported an asymptomatic nasopharyngeal carriage rate of 3.7% in the 5-24 year old population, with serogroup B in 65.7% of isolates – a grim reminder that the threat of invasive meningococcal disease is indeed just a short breath away.13,14
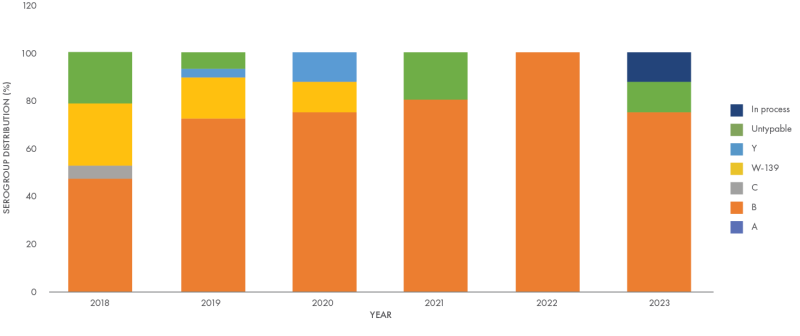
Data Source: National Reference Laboratory for Invasive Vaccine-Preventable Bacterial Diseases - Research Institute for Tropical Medicine [unpublished].
This graph has been independently created by GSK from the original data.
Novel Men B Vaccine Technology – beating the challenge of strain coverage and immunogenicity
Vaccines are serogroup-specific and while immunization against serogroups A, C, W and Y are already available locally, challenges in developing a effective vaccine against serotype B has left a gap in vaccine access.
Serogroup B has a capsule that is poorly immunogenic making capsular vaccines - which was successful in other serogroups - ineffective for MenB.15 Outer-membrane vesicle (OMV) - based vaccines were shown to be effective against single homologous MenB strain but ineffective against heterologous strains.15 These factors necessitated the employment of reverse vaccinology technology to come up with a vaccine with multiple antigenic components to enhance strain coverage.15
Impact and effectiveness of 4CMenB (Bexsero), the only Men B vaccine now available in the Philippines1-4The recombinant technology of the new GSK vaccine – with an immunogenicity that confers broad coverage across a few strains – was shown in clinical studies to have high immunogenicity and persistence even in infants.3,16 Studies also show it has an excellent tolerability profile.3,16 Furthermore, real-world data from countries like the UK, Italy, Spain, Portugal, Canada, Australia and the US have consistently shown its impact and effectiveness by offering unrivalled protection to match the threat of invasive meningococcal disease, as the first and only MenB vaccine powered with real-world evidence and faster series completion in adolescents.3,12,17-22
How to Administer 4CMenB (Bexsero) to Protect Your Patients from the Predominant MenB

Meningococcal group B vaccine (Bexsero) is approved for active immunization of individuals from 2 months of age and older against invasive meningococcal disease caused by N. meningitidis. It is available in suspension for intramuscular injections. Primary immunization of infants 2 to 5 months of age includes 3 injections of 0.5ml not less than 1 month apart, with a booster dose at 12-15 months of age. From 6 months of age onwards, the primary series consists of 2 doses with not less than 2 months interval for children below 2 years, and not less than 1 month for older children and adults. Booster doses are recommended at the 2nd year of life for infants who received primary vaccination at 6-11 months old, after 12- 23 months of the primary series in those 12 to 23 months old, and in older children and adults at continued risk of exposure.3
