3L EGFR-MET bispecific antibody in a patient with EGFR ex20ins–positive NSCLC after haemorrhagic stroke





Background, presentation and investigation
This was a 60-year-old woman with a history of diabetes and end-stage kidney disease (ESKD) requiring haemodialysis three times a week. She was admitted to the hospital in June 2022 due to shortness of breath and chest tightness lasting for 1 month. Her Eastern Cooperative Oncology Group performance status (ECOG PS) was 2. (Table)
Investigations showed primary tumour as well as pericardial effusion, pleural effusion and multiple nodules in the left lung. Cytology of the pleural fluid confirmed stage IV lung adenocarcinoma. Her carcinoembryonic antigen (CEA) level was 23.6 μg/L. (Table)
EGFR hotspot panel testing of the pleural fluid detected no mutations in exon 18 (G719A/C/S), exon 20 (S768I) and exon 21 (L861Q), and no exon 20 insertions (ex20ins). No common EGFR mutations (ie, exon 19 deletions, exon 21 L858R mutation, exon 20 T790M mutation) were found in the hotspot panel or through molecular testing.1 (Table)
Treatment before stroke
Since no EGFR mutations or other targetable lesions were detected, the patient was started on first-line chemotherapy with paclitaxel and carboplatin in early July 2022 (pemetrexed was not used as she was on renal dialysis). The pretreatment CEA level was 50.5 μg/L. She suffered from bone marrow suppression after chemotherapy and therefore carboplatin was stopped and was replaced with bevacizumab after 2 cycles.
The patient showed partial response (PR) to bevacizumab and paclitaxel. Her CEA level gradually dropped to 27.5 μg/mL in late October 2022. (Table) CT in early November 2022 revealed a paramediastinal mass in the left upper lobe of the lung (LUL) measuring 1.7 x 2.6 x 3.1 cm and multiple nodules in the lungs.
After 6 cycles, a PR was achieved and the patient continued with bevacizumab as maintenance treatment. Over 6 months of maintenance bevacizumab, her disease remained stable, and her CEA levels fluctuated between 22.2 and 26.7 μg/L. Her ECOG PS was maintained at 1. (Table)
Stroke and subsequent treatment
On 19 June 2023, during a routine haemodialysis session, the patient experienced a sudden surge in systolic blood pressure (>200 mm Hg) and loss of consciousness. Subsequent CT confirmed a large cerebellar and intraventricular haemorrhage and hydrocephalus. She underwent blood clot evacuation and right ventricular drain insertion, which provided temporary improvement, but experienced rebleeding after 5 days, necessitating another operation with left ventricular drain insertion. She was in intensive care unit for 3 weeks and stayed in hospital for a total of 6 weeks before discharge.
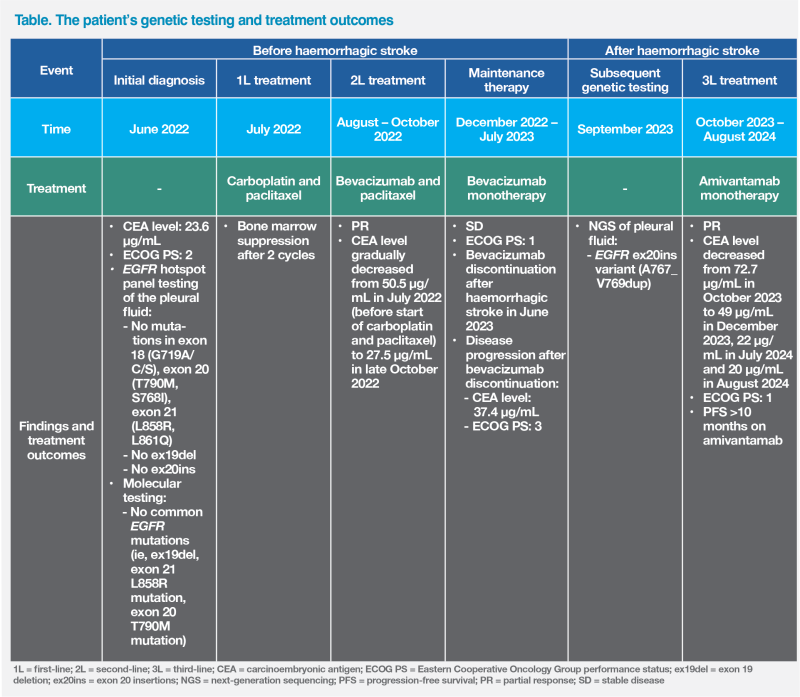
During this period, bevacizumab was stopped and her disease progressed. PET-CT thorax in September 2023 showed that the paramediastinal mass in the LUL had enlarged to 3.0 x 4.0 x 4.9 cm, while the number of lung nodules (>20) and left pleural effusion had increased. (Figure 1A) Her CEA level increased to 37.4 μg/L. The patient was mostly bedbound and required feeding assistance. Her body weight was 59 kg, and her ECOG PS deteriorated to 3. (Table)
Despite the poor PS and dismal prognosis, the patient’s family was still keen for further active treatment. After discussion with the patient’s family, next-generation sequencing (NGS) was performed in September 2023, revealing an EGFR ex20ins variant (A767_V769dup) in the pleural fluid sample. Given her genetic profile and disease history, in early October 2023, the patient was started on the EGFR-MET bispecific antibody, amivantamab, at 700 mg intravenously. Premedication with antihistamines, antipyretics, antiemetics, and glucocorticoids was given.2 (Table)
The patient achieved a PR to amivantamab. Her CEA level decreased from 72.7 μg/L in October 2023 to 49 μg/L in December 2023 and further dropped to 22 μg/L in July 2024. CT Thorax showed the primary LUL mass reduced to 3.0 x 3.3 x 3.6 cm in March 2024 and 2.9 x 3.1 x 3.4 cm in June 2024, with decrease in number of lung nodules and reduction of left pleural effusion. (Figure 1B and 1C)
During the first dose of amivantamab, the patient experienced grade 2 infusion-related reaction (IRR), necessitating temporary infusion interruption. After her symptoms improved, the infusion was resumed. Apart from IRRs during the first infusion, the patient did not experience other adverse events (AEs) with amivantamab.
The patient and her family were satisfied with the treatment. After 6 weeks, she was able to eat independently and walk at home despite residual weakness from haemorrhagic stroke. Her body weight increased to 60 kg. She attended our clinic and hospital visits in a wheelchair, with an ECOG PS of 1. She had completed 21 doses of amivantamab, with the last treatment given on 6 August 2024, and the CEA level was 20 μg/L. The patient achieved a progression-free period of more than 10 months, and amivantamab treatment will be continued.
Discussion
At our clinic, EGFR hotspot testing is a routine workup for diagnosing non-small-cell lung cancer (NSCLC). While tissue biopsy is typically preferred, it can be challenging to obtain tumour tissue in NSCLC patients with small nodules, such as ours. In these cases, malignant pleural fluid may contain high content of cell-free tumour DNA and can be more amenable to genomic analysis than both tissue and plasma samples.3
After initial negative EGFR mutation findings, our patient had PR on bevacizumab-containing treatment until experiencing haemorrhagic stroke during haemodialysis. As bevacizumab is contraindicated in patients with severe bleeding or major surgery within 28 days, treatment was discontinued.4 This resulted in disease progression shortly afterwards.
To identify targeted therapy for advanced NSCLC after haemorrhagic stroke, the patient’s family opted for NGS testing, which revealed EGFR ex20ins mutation in the pleural fluid. Consequently, our patient was started on amivantamab – the only agent targeting EGFR ex20ins in NSCLC available in Hong Kong.5
Amivantamab is an EGFR-MET bispecific antibody approved as monotherapy for advanced NSCLC with EGFR ex20ins following failure of platinum-based therapy. Its approval was based on results from the open-label, phase I CHRYSALIS trial.2,5
The CHRYSALIS trial evaluated amivantamab in 114 patients with locally advanced or metastatic EGFR ex20ins–positive NSCLC that had progressed on or after platinum‑based chemotherapy. At baseline, 99 percent of patients had ECOG PS of 0 or 1.2 Preliminary results at a median follow-up of 9.7 months showed that amivantamab was associated with an overall response rate (ORR) of 36 percent by investigator assessment and 40 percent by blinded independent central review in the efficacy population (n=81). Median progression-free survival (PFS) was 8.3 months, and median overall survival (OS) was 22.8 months.5
In a longer-term follow-up of CHRYSALIS (median, 19.2 months; n=114), 42 percent of patients remained alive. Median PFS was 6.9 months, and median OS was 23.0 months.6 These results demonstrated amivantamab’s robust efficacy and durable responses in patients with EGFR ex20ins–positive NSCLC previously treated with platinum-based chemotherapy.5,6
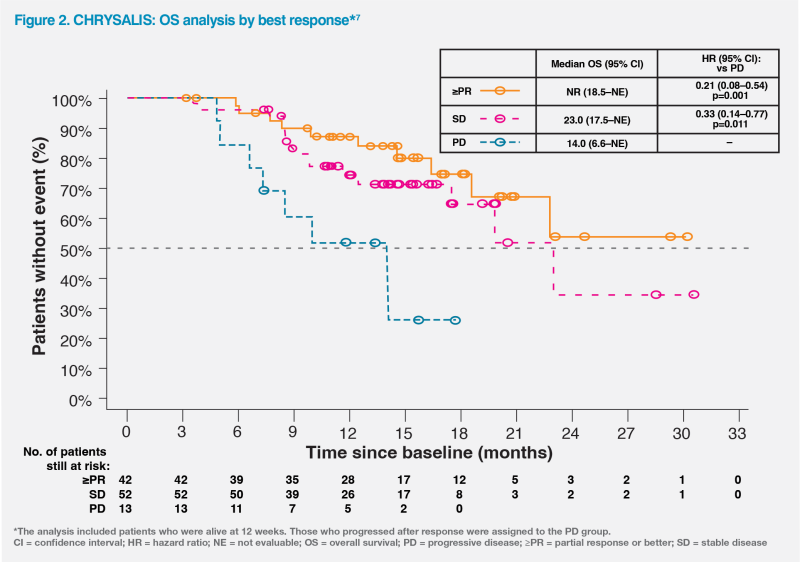
Treatment benefit with amivantamab was evident in patients with stable disease (SD) and those with ≥PR. A response-based analysis of the CHRYSALIS study showed that among patients whose disease did not progress at 12 weeks of treatment, the risk of death was reduced by 67 percent for those with SD (hazard ratio [HR], 0.33; 95 percent confidence interval [CI], 0.14–0.77; p=0.011) and by 79 percent for those with ≥PR (HR, 0.21; 95 percent CI, 0.08–0.54; p=0.001) as best response, vs those with progressive disease. These findings highlighted the value of disease control with amivantamab, irrespective of the depth of response.7 (Figure 2)
Unlike patients enrolled in CHRYSALIS, our patient had an ECOG PS of 3 and a history of diabetes, ESKD and haemorrhagic stroke. However, her response to amivantamab was better than expected. Following treatment initiation, she achieved PR with a PFS exceeding 10 months, and her quality of life substantially improved, despite residual weakness from stroke.
While our patient experienced initial IRRs during the first treatment dose, these events did not recur in subsequent infusions. She did not develop other AEs commonly associated with EGFR inhibitors.
In the CHRYSALIS trial’s safety population (n=114), the most commonly reported treatment-related AEs (TRAEs) were rash (86 percent), IRRs (66 percent) and paronychia (45 percent), which were predominantly grade 1/2 (83 percent, 63 percent, 44 percent, respectively). Grade ≥3 TRAEs occurred in 16 percent of patients, and no grade 5 TRAEs were reported. No new safety signals were found in the longer-term follow-up.5,6
In NSCLC patients with limited treatment options, further NGS testing can guide targeted therapy even if routine hotspot panel tests are negative. The patient described above had PR to and good tolerance of amivantamab despite major comorbidities and haemorrhagic stroke.