
Anti–vascular endothelial growth factor (anti-VEGF) therapy is the primary recommended treatment for patients with diabetic macular oedema (DME). At a Bayer-sponsored symposium during the 16th Congress of the Asia-Pacific Vitreo-Retina Society, experts in ophthalmology discussed clinical and real-world data on aflibercept, shared their personal experience of using it for DME treatment and highlighted its advantages vs other available anti-VEGF agents.
Aflibercept targets multiple drivers of exudative retinal diseases
Excessive activation of the VEGF signalling pathway is considered the master regulator of pathological angiogenesis in chorioretinal vascular diseases. [Prog Retin Eye Res 2021;84:100954; Nat Rev Drug Discov 2017;16:635-661; Angiogenesis 2012;15:171-185; Ophthalmologica 2021;244:93-101; EMBO Mol Med 2016;8:1265-1288]
While much was already known about the key role of VEGFR-2, recent efforts highlighted the importance of VEGFR-1 and upregulation of its family of ligands (ie, VEGF-A, VEGF-B, and placental growth factor [PlGF]) in the pathogenesis of exudative retinal diseases. [Prog Retin Eye Res 2021;84:100954; PLoS One 2018;13:e0203408]
Aflibercept binds the widest range of VEGFR-1 ligands (ie, VEGF-A, VEGF-B, PlGF, and galectin-1), which are considered key drivers of retinal diseases. The only VEGFR-1 ligand bound by brolucizumab and ranibizumab is VEGF-A, whereas faricimab also binds angiopoietin‐2. “However, to date, both preclinical and clinical data suggest no additional benefit in targeting molecular pathways besides VEGFR signalling,” said Professor Michael Stewart of the Mayo Clinic in Jacksonville, Florida, US. [Prog Retin Eye Res 2021;84:100954; Angiogenesis 2012;15:171-185; Sci Rep 2015;5:17946; Beovu Hong Kong Prescribing Information; EMBO Mol Med 2016;8:1265-1288; Transl Vis Sci Technol 2023;12:17; J Vitreoretin Dis 2023;7:8-15]
Aflibercept has a greater binding affinity to VEGF-A than brolucizumab, ranibizumab, and faricimab. [Transl Vis Sci Technol 2022;11:36; EMBO Mol Med 2016;8:1265-1288] Aflibercept also appears to have the longest intraocular half-life (9.1 days) vs brolucizumab (4.3–5.1 days), ranibizumab (7.2 days) and faricimab (7.5 days). [Transl Vis Sci Technol 2021;10:9; Retina 2020;40:643-647; Ophthalmology 2016;123:1080-1089; Mol Pharm 2020;17:695-709; Am J Ophthalmol 2012;154:682-686.e2; Vabysmo US Prescribing Information]
Together, these properties explain aflibercept’s durability, which allows for longer intervals between intravitreal injections, thereby making it a flexible treatment option for exudative retinal diseases.
Fundamentals of DME treatment with aflibercept
Anti-VEGF therapy is the standard of care for DME management. [Lancet Diabetes Endocrinol 2017;5:143-155; Clin Exp Ophthalmol 2018;46:75-86]
“Early proactive and intensive treatment, with 5–6 loading injections at monthly intervals, within the first year is crucial. Improvements may be gradual at first, and it may take a total of 8–9 injections to achieve good results. However, injection frequency can be reduced subsequently, with some anti-VEGFs, such as aflibercept, being suitable for a flexible treat-and-extend [T&E] protocol, which helps decrease the treatment burden,” said Professor Tien-Yin Wong of Tsinghua Medicine, Tsinghua University, Beijing, China and the Singapore National Eye Centre, Singapore. [Clin Exp Ophthalmol 2018;46:75-86; Eylea Hong Kong Prescribing Information]
T&E regimen with aflibercept
When used as a T&E regimen, aflibercept offers the flexibility to individualize treatment, allowing injection intervals of up to Q16W from 1 year onwards. As demonstrated in several DME studies, aflibercept’s T&E regimen achieves robust vision outcomes while reducing the treatment burden. [J Chin Med Assoc 2022;85:246-251; Sci Rep 2020;10:22030; Ophthalmologica 2020;243:255-262; Sci Rep 2021;11:4488] In a prospective, open-label, single-arm, nonrandomized trial, 66.7 percent of DME patients treated with aflibercept were on Q16W dosing at the end of the 2-year trial period. [Sci Rep 2021;11:4488]
The phase III, noninferiority VIOLET study (n=458) was one of several randomized controlled trials (RCTs) that demonstrated the efficacy and safety of T&E dosing with aflibercept in patients with DME. [Adv Ther 2022;39:2701- 2716]
In the trial, the researchers investigated whether intravitreal aflibercept administered either as a T&E or pro re nata (PRN) regimen provided similar efficacy and safety as fixed Q8W dosing in patients with DME who had previously completed ≥1 year of aflibercept treatment. Results showed that the flexible aflibercept regimens achieved similar functional outcomes to fixed Q8W dosing at week 52 (primary endpoint), with best-corrected visual acuity (BCVA) changes from baseline of +0.5 letters with T&E, +1.7 letters with PRN and +0.4 letters with fixed Q8W dosing (p<0.0001 for noninferiority tests vs fixed dosing regimen). The results were maintained in the long term (ie, week 100). (Figure 1)
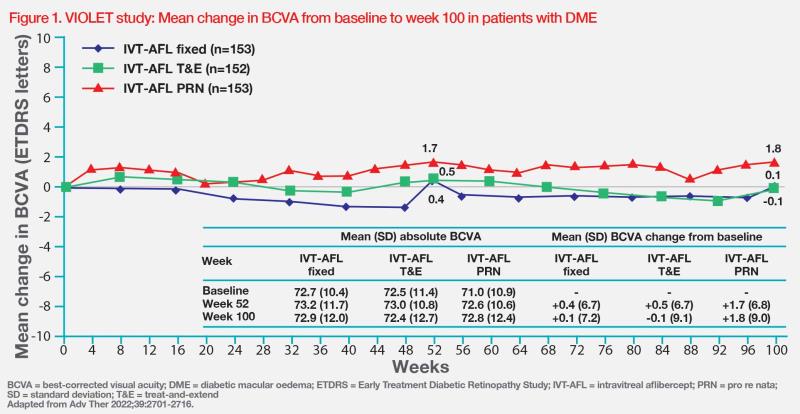
Aflibercept T&E regimen was associated with the lowest treatment burden (T&E: 10.0 injections and 13.3 clinic visits; PRN: 11.5 injections and 25.0 clinic visits; fixed dosing regimen: 12.3 injections and 16.1 clinic visits) and its safety profile in VIOLET was consistent with previous studies, with no new safety signals and comparable incidence of adverse events (AEs) reported between all treatment groups.
Does fluid affect visual outcomes?
While anatomical outcomes such as presence or absence of fluid are important considerations in management of exudative retinal diseases, its role in DME is currently not yet well-defined. In a post hoc analysis of the DRCR Retina Network Protocol T, changes in optical coherence tomography (OCT) central subfield thickness (CST) were found to account for only a small proportion of the total variation in VA changes in patients with DME who received either aflibercept, bevacizumab or ranibizumab. [JAMA Ophthalmol 2019;137:977-985]
Similar results were also reported in post hoc analysis of VISTA and VIVID, two phase III RCTs in patients with DME treated with intravitreal aflibercept injections. Results showed significant vision improvements through week 100 with continued treatment in a small number of eyes despite limited early anatomic response. [Ophthalmol Retina 2018;2:558-566]
“Thus, there is still limited correlation between anatomic response and visual outcomes in patients with DME,” Wong noted.
Aflibercept achieves RCT-like gains in real world
“It has been shown that real-world patients with DME are able to achieve and maintain outcomes comparable to those in RCTs when treated with aflibercept,” said Professor Gavin Tan of the Singapore National Eye Centre, Singapore.
VISTA and VIVID RCTs compared outcomes of patients (n=872) with DME who received intravitreal aflibercept injections or macular laser photocoagulation. In patients who received aflibercept Q8W, mean BCVA improvements from baseline ranged from +9.4 to +11.7 letters throughout the 3-year follow-up period despite fewer injections after year 1 (ie, mean number of yearly aflibercept injections, 4.6–5.1), vs +0.2 to +1.6 letters among laser-treated controls. [Ophthalmology 2014;121:2247-2254; Ophthalmology 2015;122:2044-2052; Ophthalmology 2016;123:2376-2385]
Similar continued visual gains were observed in a retrospective, real-life study that followed treatment-naïve patients with DME who received intravitreal aflibercept injections for 3 years. Patients received the recommended five initial loading intravitreal aflibercept injections at monthly intervals, followed by a PRN regimen. After a mean BCVA gain of +11.2 letters from baseline in the first year, patients continued to experience visual improvements from baseline, despite fewer mean injections, after 2 years (+6.2 letters, 2.9 injections) and 3 years (+6.9 letters, 2.6 injections). [Eur J Ophthalmol 2021;31:1201-1207]
Other real-world prospective studies of aflibercept showed comparable results, with continued vision gains in both treatment-naïve and previously treated patients with DME, despite receiving fewer intravitreal aflibercept injections than the label-recommended regimen. [Sci Rep 2022;12:18242; Int J Retina Vitreous 2022;8:52] For instance, in a retrospective, real-life, cohort analysis of 99 aflibercept-treated eyes of treatment-naïve diabetic patients in the UK, who received a mean number of 6.92 intravitreal injections, the mean visual acuity has increased from 59.7 Early Treatment Diabetic Retinopathy Study (ETDRS) letters to 69.6 letters at 12 months. At the same time, 33.67 percent of aflibercept-treated eyes gained ≥15 ETDRS letters. [Eur J Ophthalmol 2020;30:557-562]
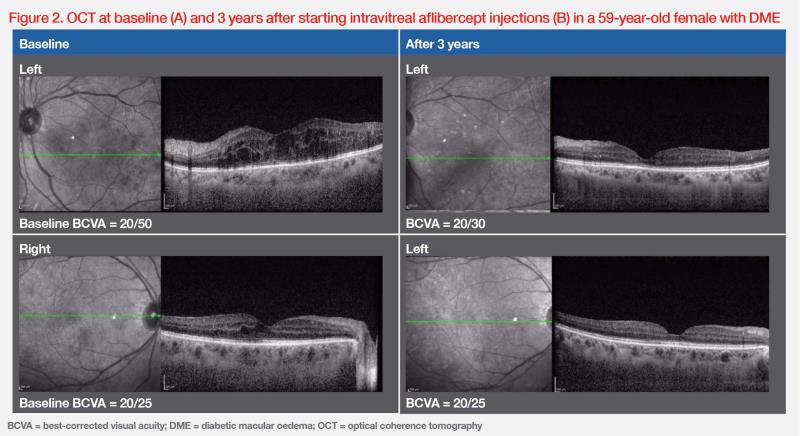
“In our clinical experience, treatment schedules of patients with DME are often less than ideal in the real world, with treatment delays due to missed appointments being a common scenario,” shared Tan. “Nevertheless, the durable vision gains achieved with aflibercept in RCTs are still seen in actual clinical practice, as demonstrated by our patient with DME treated with an aflibercept T&E regimen. Having missed a number of appointments and deferred several visits, the patient received a total of 13 and 11 injections to the right and left eye, respectively, over a course of 3 years.” (Figure 2)
Summary
Among available anti-VEGF agents, aflibercept targets the most diverse range of ligands in the VEGFR signalling pathway that is central to DME pathogenesis. RCTs and real-world evidence demonstrate robust and durable vision gains and reduced treatment burden with aflibercept T&E regimen, which allows treatment intervals of up to Q16W.