Acute and preventive migraine management with CGRP-targeted therapies











At an industry-sponsored meeting during the 10th Congress of the European Academy of Neurology (EAN 2024), Professor Marja-Liisa Sumelahti of Tampere University in Tampere, Finland, Dr Roni Sharon of the Tel Aviv University in Tel Aviv, Israel, and Dr Margarita Sánchez del Rio of the Clínica Universidad de Navarra in Madrid, Spain, discussed the role of calcitonin gene-related peptide (CGRP)–targeted agents, specifically the oral CGRP receptor antagonist (RA), rimegepant, in early migraine management.
Unmet needs and opportunities
“Migraine affects over 1 billion people globally,” noted Sumelahti. “Despite advances in migraine care, unmet needs remain. These include insufficient response to triptans that may lead to poorer outcomes vs responders, as well as low adherence rates due to poor efficacy and adverse events [AEs].” [Lancet 2021;397:1485-1495; BMC Neurol 2021;21:425; Cephalalgia 2021;41:894-904]
For instance, the online, noninterventional, cross-sectional OVERCOME EU survey showed that >70 percent of patients on triptans reported poor or very poor satisfaction with acute triptan treatment. [Pain Ther 2024;13:589-607]
“Triptans [eg, sumatriptan and rizatriptan] may be unsuitable for a number of patients due to associated AEs, contraindications [eg, coronary/ischaemic heart disease, moderate-to-severe/uncontrolled or untreated mild hypertension, history of stroke/transient ischaemic attack], or insufficient response,” said Sumelahti. [Headache 2014;54:278-289; NeurolTher 2022;11:167-183; Pain Ther 2021;10:415-432; Cephalalgia 2023;43:3331024221141686] “Health-related quality of life and work productivity are significantly impaired in patients with insufficient response to triptans.” [J Headache Pain 2020;21:41]
“There is also an opportunity to improve migraine prevention strategies, as according to a cross-sectional questionnaire-based survey in 10 European countries, only 1.6–13.7 percent of eligible patients use preventive migraine treatments,” she added. [J Headache Pain 2018;19:10]
Defining effective migraine treatment
The 2022 European Headache Federation consensus considers treatment of migraine attacks effective if it restores a patient’s well-being (ie, improvement of headache to mild/absent, relief of nonpain symptoms, and absence of AEs) within 2 hours and maintains it for ≥24 hours. [J Headache Pain 2022;23:133]
“It is important to address ineffective treatments to minimize the risk of progression from episodic to chronic migraine. Notably, medication overuse headache [MOH], associated with regular overuse of acute headache medication, is a risk factor for migraine progression,” stressed Sharon. [Neurology 2015;84:688-695; Cephalalgia 2018;38:1-211; J Neurol 2023;270:5692-5710]
Rimegepant in early migraine management
Rimegepant is a CGRP-targeted therapy indicated for both acute and preventive treatment of migraine. [Nat Rev Neurol 2018;14:338-350; Nurtec Hong Kong Prescribing Information, November 2022]
Acute therapy
Study 303 was a double-blind, randomized, placebo-controlled, phase III trial that demonstrated the efficacy and safety of a single dose of oral rimegepant (75 mg) for acute migraine treatment. Among adults aged ≥18 years with a migraine history of ≥1 year, rimegepant was superior vs placebo in both pain relief (21 vs 11 percent; p<0.0001) and freedom from the most bothersome symptom (MBS; 35 vs 27 percent; p=0.0009) at 2 hours postdose. Tolerability was similar to placebo, with no safety concerns. [Lancet 2019;394:737-745; NCT03461757]
“These results may help with treatment adherence and encourage patients to take their medications earlier, such as during the aura phase, because of rimegepant’s preventive indication, unlike triptans,” Sharon commented. [Nat Rev Neurol 2018;14:338-350; Nurtec Hong Kong Prescribing Information, November 2022]
Post-hoc analysis of Study 201, a long-term, open-label safety study, also showed that in patients who received rimegepant 75 mg up to once daily PRN for up to 52 weeks, overall mean monthly migraine days (MMD) decreased from 10.9 at baseline to 8.9 by week 52, translating to approximately 24 fewer migraine days per year. [J Headache Pain 2022;23:10]
Pooled results from three phase III trials that assessed rimegepant’s acute treatment efficacy (ie, pain relief at 2 hours postdose) based on triptan treatment experience in 3,507 adults were significantly in favour of rimegepant vs placebo regardless of prior or current triptan use and response. Rimegepant was effective in the coprimary endpoints of pain freedom and MBS freedom at 2 hours postdose in all subgroups (p≤0.013), except for MBS freedom in the triptan-naïve group (p=0.06).There were no differences in coprimary endpoints in pairwise comparisons of rimegepant-treated participants. [Cephalalgia 2023;43:3331024221141686]
Preventive therapy
“Position statements of international societies recognize that CGRP-targeted therapies should be considered as first-line options for migraine prevention,” shared Sánchez-Del-Rio. [J Headache Pain 2022;23:67; Headache 2024;64:333-341] “This is a crucial update because it relieves both patients and healthcare systems of the burden associated with treatment failures and inadequate responses to other therapies, offering potentially effective options from the beginning.” [J Headache Pain 2023;24:56]
“Additionally, the oral route is often preferred by patients vs injections,” she noted. [J Headache Pain 2017;18:102] Rimegepant offers a convenient oral administration route with a favourable pharmacokinetic profile (eg, short half-life of around 11 hours), providing greater flexibility vs CGRP monoclonal antibodies (mAbs) (eg, may allow prompt discontinuation due to planned or unplanned pregnancy and AEs). [Lancet 2021;397:51-60]
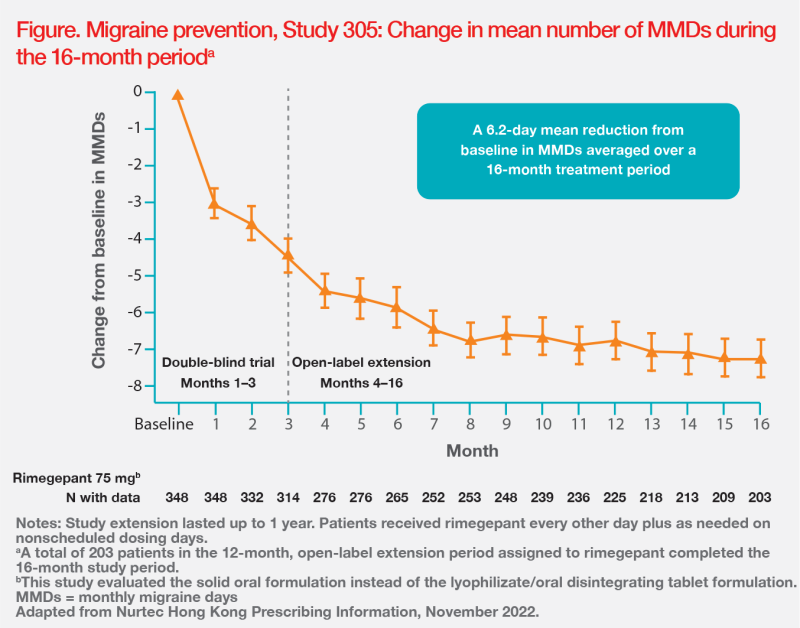
Rimegepant’s efficacy and safety in prophylactic treatment of migraine are supported by results of the randomized, double-blind, placebo-controlled, phase II/III Study 305. The study included adults (n=747) with a migraine history of ≥1 year. Participants received either oral rimegepant 75 mg or matching placebo every other day for 12 weeks (double-blind treatment phase). Results showed that rimegepant was superior vs placebo in terms of change in mean number of total MMD in weeks 9–12 (-4.3 vs -3.5 days; p=0.0099). [Lancet 2021;397:51-60]
Study 305 participants were allowed to continue treatment in an open-label extension study for an additional 12 months. Efficacy results were sustained for up to 1 year. (Figure) [Nurtec Hong Kong Prescribing Information, November 2022]
Addressing tolerability concerns
Since CGRP has a physiologic role in peristaltic activity of the small and large intestines, real-world surveys have shown that constipation is a major AE associated with CGRP-targeted agents, specifically the mAbs erenumab, fremanezumab and galcanezumab. [Front Physiol 2022;12:820006]
“However, we do not see this with rimegepant because of its shorter half-life vs mAbs and every-other-day preventive dosing regimen,” said Sharon. [Front Physiol 2022;12:820006; Lancet 2021;397:51-60; Nurtec Hong Kong Prescribing Information, November 2022] “Meanwhile, we have safely combined mAbs with gepants in our clinical practice in the US. This has resulted in about 20–30 percent improvement in terms of efficacy, without any compounded side effects.”
Summary
CGRP-targeted therapies are effective and well-tolerated in acute and preventive treatment of migraine, and are recommended as first-line options for migraine prevention. Rimegepant, in particular, offers a convenient oral administration route and favourable pharmacokinetic profile, with efficacy and tolerability demonstrated in both the acute treatment and prevention settings.