
Heterogeneity in severe asthma
Exacerbation remains common despite recent advances in severe asthma treatment. An analysis of the International Severe Asthma Registry (n=4,990) demonstrated that in adult patients on Global Initiative for Asthma (GINA) Step 5 treatment or those with uncontrolled disease despite GINA Step 4 treatment (n=4,823), 40.9 percent experienced ≥1 asthma exacerbation a year. [Chest 2020;157:790-804] In a systematic review of 20 clinical reports, most biologics demonstrated suboptimal efficacy in reducing exacerbation among patients with blood eosinophil count (BEC) <300 cells/μL. [Adv Ther 2023;40:2944-2964]
Patients with severe asthma often exhibit diverse phenotypic patterns of inflammation markers. A Hong Kong study (n=232) of adult patients with severe asthma receiving GINA Step 4 or 5 treatment showed that 72.8 percent had T-helper 2 (Th2) phenotype, as indicated by elevated levels of blood eosinophil and/or immunoglobulin E (IgE), while 29.7 percent had an overlapping phenotype with eosinophilia and elevated IgE. [J Asthma Allergy 2023:16:173-182]
“Th2 marker levels are dynamic. Each patient can exhibit variability over time,” noted Burhan. In a 5-year Chinese study (n=241), 61.9 percent of severe asthma patients had BECs traversing the threshold of 300 cells/μL, while 35.3 percent had fractional exhaled nitric oxide (FeNO) repeatedly exceeding the threshold of 25 ppb. [World Allergy Organ J 2021;14:100547]
TSLP in asthma pathophysiology
TSLP, together with other epithelial cytokines (eg, interleukin [IL]-25 and IL- 33), are released by airway epithelial cells upon exposure to allergens or inhaled environmental insults, such as bacteria, viruses, and smoke. These epithelial cytokines are key drivers of Th2 inflammation that result in diverse innate and adaptive immune responses. (Figure 1) [Expert Opin Ther Targets 2020;24:777-792; J Clin Invest 2019;129:1441-1451]
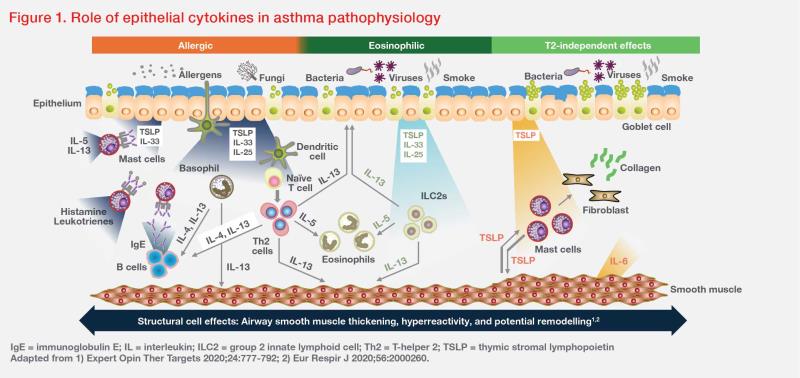
For example, TSLP may synergize with IL-25 or IL-33, inducing group 2 innate lymphoid cells to produce Th2 cytokines (eg, IL-5, IL-13), which in turn leads to eosinophilic inflammation. Additionally, TSLP-activated dendritic cells may induce T-cell differentiation and cytokine release, leading to mucus hypersecretion in airway tissue and smooth muscle contraction resulting in AHR. TSLP may also contribute to airway remodelling in different asthma inflammation endotypes through Th2-independent effects involving smooth muscle cells, mast cells and lung fibroblasts. (Figure 1) [Expert Opin Ther Targets 2020;24:777-792; Respir Res 2020;21:268]
“[As TSLP is an upstream cytokine initiating myriad responses in asthma,] blocking TSLP has become a potential therapeutic approach,” Burhan highlighted.
Tezepelumab’s role in SUA
Tezepelumab is a human mAb that blocks TSLP from binding to its receptor. This blockade leads to inhibition of inflammatory cytokine production and immune cell activation in multiple asthmarelated inflammation pathways. [Respir Res 2020;21:268]
AAER reduction
The phase II, randomized, placebo-controlled, dose-ranging PATHWAY trial evaluated the efficacy and safety of tezepelumab in 550 patients with SUA despite treatment containing medium-to-high doses of inhaled glucocorticoids. Over 52 weeks, subcutaneous (SC) medium-dose tezepelumab (210 mg Q4W) demonstrated a 71 percent reduction in annualized asthma exacerbation rate (AAER) vs placebo (AAER, 0.20 vs 0.72; 90 percent confidence interval [CI] for relative AAER reduction, 54–82; p<0.001). [N Engl J Med 2017;377:936-946]
The phase III, randomized, placebo-controlled NAVIGATOR trial investigated the effect of SC tezepelumab 210 mg Q4W in SUA patients on inhaled glucocorticoids (fluticasone propionate ≥500 μg/ day or equivalent) for ≥12 months plus ≥1 other controller with or without oral glucocorticoids for ≥3 months. Endpoint assessment was conducted in 1,059 patients (mean age, 49.5 years; male, 36.5 percent; oral glucocorticoids, 9.4 percent), including 528 patients in the tezepelumab group and 531 patients in the placebo group. Over 52 weeks, tezepelumab significantly reduced AAER by 56 percent vs placebo (AAER, 0.93 vs 2.10; 95 percent CI for relative AAER reduction, 47–63; p<0.001). [N Engl J Med 2021;384:1800-1809]
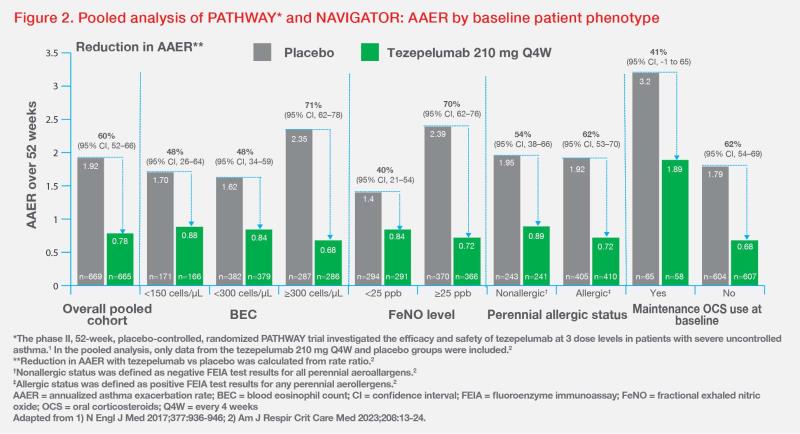
Tezepelumab also showed clinically meaningful reduction in AAER across subgroups, including patients with different BEC or FeNO levels, perennial aeroallergen sensitivity, and maintenance oral corticosteroid (OCS) use at baseline, according to a post-hoc analysis of pooled data from PATHWAY and NAVIGATOR. (Figure 2) [Am J Respir Crit Care Med 2023;208:13-24]
When categorized by multiple biomarkers, tezepelumab-treated patients with BEC ≥300 cells/μL and FeNO ≥25 ppb or perennial allergy achieved 71–77 percent reduction in AAER vs placebo. Notably, in the triple Th2-high subgroup with BEC ≥300 cells/μL, FeNO ≥45 ppb and perennial allergy, tezepelumab demonstrated an 80 percent reduction in AAER vs placebo. (Figure 3) [Am J Respir Crit Care Med 2023;208:13-24; AstraZeneca, data on file 2022]
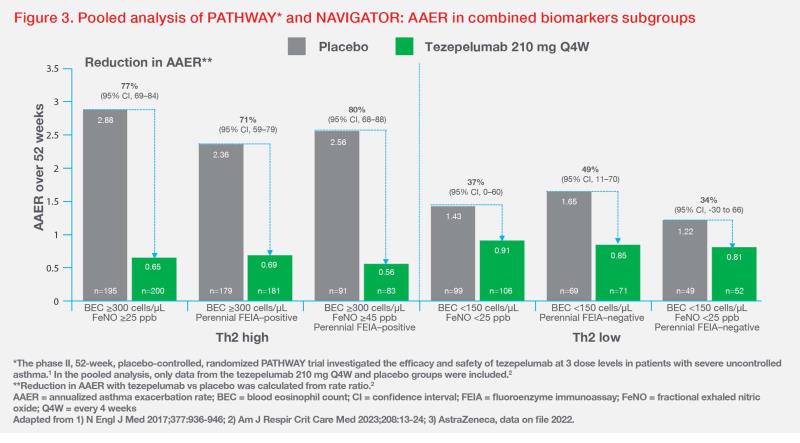
“Tezepelumab may also reduce AAER in patients with Th2-low disease, a severe asthma subtype with limited treatment options,” Burhan highlighted. In the triple Th2-low subgroup with BEC <150 cells/μL and FeNO <25 ppb without perennial allergy, tezepelumab reduced AAER by 34 percent vs placebo. (Figure 3) [Am J Respir Crit Care Med 2023;208:13-24]
Tezepelumab also demonstrated efficacy in SUA patients with comorbid nasal polyps (NPs). In an exploratory subgroup analysis of NAVIGATOR, 118 patients with a 2-year history of NPs before trial enrolment achieved an 85 percent reduction in AAER with tezepelumab vs placebo (0.43 vs 2.87). Improvement in Sino- Nasal Outcome Test–22 total score was also seen with tezepelumab vs placebo (least-squares mean [LSM] change at 52 weeks from baseline, -21.06 points vs -10.48 points; LSM difference, -10.58 points). [J Asthma Allergy 2023;16:915-932]
AHR and mucus plug reductions
In an exploratory analysis of the phase II CASCADE trial in 116 patients with moderate-to-severe uncontrolled asthma, tezepelumab significantly reduced AHR to mannitol vs placebo, as assessed by LSM change from baseline in interpolated or extrapolated provoking dose of mannitol required to induce ≥15 percent reduction in forced expiratory volume in 1 second [FEV1] from baseline (197.4 mg vs 58.6 mg; difference, 138.8; pnominal=0.030). [Lancet Respir Med 2021;9:1299-1312]
In a prespecified analysis of CASCADE, tezepelumab was associated with reduction in occlusive mucus plugs among 82 patients who completed ≥20 weeks of treatment and had CT scans both at baseline and a visit within 8 weeks after the last treatment. Mucus plug scores on CT scan were lower among patients in the tezepelumab vs placebo group (absolute mean change from baseline, -1.7 vs 0.0). Additionally, more patients in the tezepelumab vs placebo group transitioned from having ≥1 mucus plug to having none (37.8 percent vs 13.3 percent). [NEJM Evid 2023;doi:10.1056/ EVIDoa2300135]
HRQoL
“With sustained improvements in asthma control and symptoms, patients receiving tezepelumab are more likely to have better health-related quality of life [HRQoL],” said Burhan.
In the NAVIGATOR trial, tezepelumab demonstrated HRQoL improvement vs placebo, as assessed by Asthma Control Questionnaire–6 (ACQ-6; -1.55 vs -1.22; difference, -0.33; p<0.001), Asthma Symptom Diary (ASD; -0.71 vs -0.59; difference, -0.12; p=0.002), Asthma QoL Questionnaire (standardized) for patients aged ≥12 years (1.49 vs 1.15; difference, 0.34; p<0.001), and St George’s Respiratory Questionnaire (-21.91 vs -15.86; LSM difference, -6.05). [N Engl J Med 2021;384:1800-1809; Am J Respir Crit Care Med 2022;205:A2360]
Safety
Tezepelumab was well tolerated, according to pooled analysis of the PATHWAY and NAVIGATOR. The most common adverse events were nasopharyngitis (19.5 percent for tezepelumab vs 19.1 percent for placebo), upper respiratory tract infection (9.3 percent vs 13.3 percent), headache (7.8 percent vs 7.5 percent) and asthma (7.4 percent vs 15.7 percent). Rates of injection-site reactions were 4 percent (n=25) with tezepelumab vs 3 percent (n=21) with placebo. Anaphylactic reactions were observed in none of the patients in the tezepelumab group vs one patient (0.1 percent) in the placebo group. No on-treatment deaths were reported. [Am J Respir Crit Care Med 2023;208:13-24]
Tezepelumab’s position in GINA 2023 GINA’s 2023
Report recommends add-on anti-TSLP treatment (ie, SC tezepelumab) for patients aged ≥12 years with severe asthma (Evidence A). Anti-TSLP treatment may also be considered in patients without elevated Th2 markers. [GINA 2023 Report, https:// www.ginasthma.org]
“Tezepelumab can be a viable option for asthma patients, regardless of Th2 marker levels,” Burhan commented. “When considering tezepelumab use in patients with low Th2 disease, it is necessary to confirm asthma diagnosis.”
