
Respiratory syncytial virus (RSV) infection is a common cause of acute lower respiratory tract disease (LRTD) and a leading cause of hospitalization in infants. In Hong Kong, the single-dose RSV prefusion F protein–based (RSVpreF) vaccine, indicated for active immunization of pregnant individuals at a gestational age of 32–36 weeks, is now available for prevention of LRTD and severe LRTD caused by RSV in infants ≤6 months of age. This article discusses the burden of RSV infections and the efficacy and safety of the RSVpreF vaccine specifically against medically attended severe RSV-associated LRTD in infants, which demonstrated an efficacy of 81.8 percent at 90 days after birth and 69.4 percent at 180 days after birth in a pivotal trial.
Burden of RSV infections in infants
Although RSV infection is often associated with mild symptoms, it can cause severe LRTD requiring hospitalization in infants and older individuals. [https:// www.cdc.gov/rsv/about/index.html]
In infants, RSV hospitalization is considerably more likely in the first 6 months of life, regardless of gestational age at birth. In the US, >75 percent of RSV hospitalizations in infants occur at or before the age of 6 months. [Am J Perinatol 2017;34:51-61; Early Hum Dev 2015;91:541-546; Pediatrics 2013;132:e341-e348; J Infect Dis 2022;226(Suppl 2):S154-S163; JAMA Netw Open 2024;7:e2416077]
In Hong Kong, RSV is the leading cause of hospitalization due to common respiratory viruses in children aged <1 year. From 1998 to 2012, the rate of RSV-associated hospitalization in this age group was more than two times higher vs hospitalization associated with influenza or parainfluenza viruses (51 vs 22 and 22 percent, respectively). [Viruses Medicine (Baltimore) 2015;94:e2024]
Observational, case-control studies and meta-analyses showed that RSV-associated LRTD increases the long-term risk of wheezing and asthma in children. [Open Forum Infect Dis 2023;10:ofad450; Lancet 2023;401:1669-1680; J Infect Dis 2022;226(Suppl 1):S38-S44; Pediatr Infect Dis J 2013;32:820-826] In the 2010 Global Burden of Disease Study, RSV was associated with 6.7 percent of post-neonatal deaths (ie, at 28–364 days of life), which accounted for 33.3 percent of all LRTD deaths in this age group. [Lancet 2012;380:2095-2128]
Treatment for RSV infection is primarily supportive. Specific antiviral agents have demonstrated modest efficacy and are mainly reserved for immunocompromised individuals. [J Pediatr Pharmacol Ther 2009;14:75-85; Lancet 2022;400:392-406]
These findings underscore the importance of preventive strategies to reduce the burden of RSV-associated LRTD in infants.
Maternal RSVpreF vaccine for RSV-associated LRTD prevention in infants
RSV is comprised of two major antigenic subgroups, A and B, both of which may cause severe disease and contribute to the global RSV burden. [Int J Infect Dis 2020;90:5-17; Infect Dis Ther 2024;13:1725-1742]
The RSVpreF vaccine, a single-dose bivalent vaccine against RSV A and B, is now available in Hong Kong for active immunization of pregnant individuals at 32–36 weeks’ gestational age to prevent both LRTD and severe LRTD caused by RSV in infants aged ≤6 months. It is also approved for prevention of RSV-associated LRTD in individuals aged ≥60 years. [https://www.pfizer.com. hk/en/news-en/pfizer%E2%80%99s-first-dual-indication-vaccine-for-respiratory-syncytial-virus-rsv-is-available-this-month-in-hong-kong/; RSVpreF vaccine Hong Kong Prescribing Information, March 2024]
Approval of the RSVpreF vaccine’s maternal immunization indication is based on results of the phase III, double-blind, randomized, placebo-controlled MATISSE trial conducted in 18 countries, which examined the efficacy and safety of RSVpreF vaccination in women with an uncomplicated singleton pregnancy. [N Engl J Med 2023;388:1451-1464]
MATISSE study background
The study included a total of 7,357 pregnant individuals aged ≤49 years (median, 29 years) at a gestational age of 24–36 weeks (median, 31.3 weeks) who received a single intramuscular (IM) injection of either the RSVpreF vaccine 120 μg (n=3,682) or placebo (n=3,676). Overall, 12.5 percent of the maternal participants were Asian (n=918). [N Engl J Med 2023;388:1451-1464]
The two primary efficacy endpoints were medically attended severe RSV-associated LRTD and medically attended RSV-associated LRTD in infants within 90, 120, 150 and 180 days after birth. These were assessed in a total of 7,128 infants (RSVpreF vaccine group, n=3,570; placebo group, n=3,558). A lower boundary of confidence interval (CI) for vaccine efficacy (99.5 percent CI at 90 days; 97.58 percent CI at later intervals) >20 percent was considered to meet the success criterion for vaccine efficacy with respect to the primary endpoints.
MATISSE efficacy and safety results
At prespecified interim analysis, RSVpreF vaccine demonstrated efficacy vs placebo in preventing medically attended severe RSV-associated LRTD in infants. Within 90 days after birth, vaccine efficacy against medically attended severe LRTD was 81.8 percent (99.5 percent CI, 40.6–96.3). Within 180 days after birth, corresponding vaccine efficacy was 69.4 percent (97.58 percent CI, 44.3–84.1). These results met the statistical success criterion (ie, lower boundary of CI >20 percent) for decreasing the incidence of medically attended severe RSV-associated LRTD among infants through 180 days after birth. (Figure) [N Engl J Med 2023;388:1451-1464]
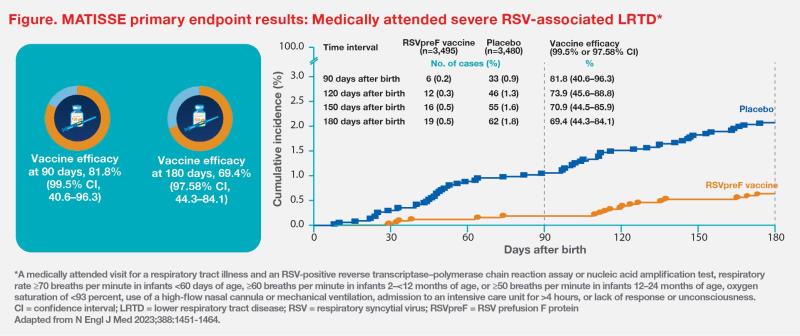
Medically attended RSV-associated LRTD also occurred at significantly lower rates in the vaccine vs placebo group at 120, 150 and 180 days after birth (vaccine efficacy, 56.8 percent [97.58 percent CI, 31.2–73.5], 52.5 percent [97.58 percent CI, 28.7–68.9] and 51.3 percent [97.58 percent CI, 29.4–66.8], respectively).
The RSVpreF vaccine was well tolerated in the MATISSE trial. Among maternal participants, most reactogenicity events within 7 days were mild to moderate in severity; the most common local reaction was injection-site pain (41 vs 10 percent for RSVpreF vaccine vs placebo), while the most common systemic events were muscle pain (27 vs 17 percent) and headache (31 vs 28 percent). The incidence of adverse events (AEs) reported within 1 month after injection or within 1 month after birth was similar between the vaccine group (13.8 percent of women and 37.1 percent of infants) and the placebo group (13.1 and 34.5 percent, respectively). [N Engl J Med 2023;388:1451-1464]
RSVpreF vaccination recommendations
The US Centers for Disease Control and Prevention (CDC) advises that the RSVpreF vaccine can be administered to pregnant individuals along with other vaccines recommended during pregnancy (eg, tetanus, diphtheria and pertussis [Tdap] vaccine, influenza vaccine, and COVID-19 vaccine), without regard to timing, including simultaneous vaccination at different anatomic sites on the same day. Additional doses are not recommended during future pregnancies, although this may change as more data become available. [https://www. cdc.gov/vaccines/vpd/rsv/hcp/pregnant-people-faqs.html; MMWR Morb Mortal Wkly Rep 2023;72:1115-1122]
The RSVpreF vaccine has not been associated with an increased risk of preterm births or adverse perinatal outcomes when administered at a gestational age of 32–36 weeks. [JAMA Netw Open 2024;7:e2419268] The US Advisory Committee on Immunization Practices (ACIP) judged the benefits of maternal RSVpreF vaccination during this dosing interval to outweigh the potential risks for preterm birth and hypertensive disorders of pregnancy. [MMWR Morb Mortal Wkly Rep 2023;72:1115-1122]
Summary
Preventive strategies against RSV infection are crucial due to the substantial burden of RSV-associated LRTD in infants. The single-dose bivalent RSVpreF vaccine has demonstrated efficacy and a favourable safety profile when administered late in pregnancy and is now approved for maternal active immunization to prevent LRTD and severe LRTD caused by RSV in infants ≤6 months of age.