
Respiratory syncytial virus (RSV) is an important cause of severe respiratory illness, often leading to hospitalization and/or death in older individuals. A bivalent (RSV A and RSV B) RSV prefusion F protein–based (RSVpreF) vaccine is now available in Hong Kong for prevention of lower respiratory tract disease (LRTD) caused by RSV in individuals ≥60 years of age. This article highlights the single-dose vaccine’s sustained protective efficacy through two full RSV seasons, with cumulative vaccine efficacy against RSV-associated LRTD with ≥3 signs or symptoms exceeding 80 percent.
Burden of RSV in older individuals
RSV infection is a major cause of LRTD, particularly among older individuals, infants, and young children. In individuals ≥60 years of age, the risk of severe RSV disease is highest in those with chronic underlying medical conditions (eg, lung diseases, cardiovascular diseases, immune compromise, diabetes, kidney or liver disorders) or other factors such as frailty, advanced age, or residence in a nursing home or other long-term care facility. [https://www.who.int/ teams/health-product-policy-and-standards/standards-and-specifications/vaccine-standardization/respiratory-syncytial-virus-disease; MMWR Morb Mortal Wkly Rep 2023;72:793-801]
The RSV-NET, a population-based surveillance system that monitors RSV hospitalizations in the US, reported that individuals ≥65 years old hospitalized with RSV had higher prevalence of coronary artery disease (2.0-fold), chronic obstructive pulmonary disease (COPD; 3.4-fold), diabetes (1.6-fold), and asthma (2.2-fold) compared with the same-age cohort in the general population. [https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-10- 25-26/03-Patton-Adult-RSV-508.pdf]
Findings from a prospective, multicentre study in the US that compared in-hospital outcomes among individuals aged ≥60 years hospitalized with RSV (n=304), COVID-19 (n=4,734), or influenza (n=746) showed that those infected with RSV had more severe disease in terms of receipt of standard flow oxygen (adjusted odds ratio [aOR], 2.97 [vs COVID-19] and 2.07 [vs influenza]), high-flow nasal cannula/noninvasive ventilation support (aOR, 2.25 [vs COVID-19] and 1.99 [vs influenza]), intensive care unit admission (aOR, 1.49 [vs COVID-19] and 1.55 [vs influenza]), and invasive mechanical ventilation/death (aOR, 1.39 [vs COVID-19] and 2.08 [vs influenza]). [MMWR Morb Mortal Wkly Rep 2023;72:1083-1088]
RSV infection can also negatively impact long-term survival. An observational retrospective cohort study evaluated individuals aged ≥60 years hospitalized with RSV (n=645) or influenza A/B (n=1,878) infection during five consecutive seasons. The 1-year survival rate after admission was significantly lower (74.2 vs 81.2 percent; p<0.001) in patients hospitalized with RSV vs influenza (odds ratio [OR], 1.3; 95 percent confidence interval [CI], 1.0–1.6; p=0.019). Almost one-third of hospitalized RSV patients aged ≥75 years died within 1 year of admission (survival rate, 68.4 percent). [Clin Infect Dis 2019;69:197- 203; J Infect Dis 2020;222:1298-1310]
Burden in HK
Excluding SARS-CoV-2, RSV remains a leading respiratory pathogen in Hong Kong, following type A influenza. [https://www.chp.gov.hk/en/statistics/data/10/641/642/2274.html]
A retrospective study that reported annual hospitalization rates for respiratory viral infections during a 15-year period (1998–2012) showed that among older individuals aged ≥65 years, influenza A ranked top (18.3/10,000 persons), followed by RSV (5.7/10,000 persons). [Medicine 2015;94:e2024] Another retrospective cohort study revealed that >70 percent of adults (mean age, 75 years) admitted to the hospital with RSV had lower respiratory tract complications, such as pneumonia (42.3 percent), bronchitis (21.9 percent), or exacerbations of COPD/ asthma (27.3 percent). Mortality rates at 30 days and 60 days were 9.1 percent and 11.9 percent, respectively – similar to those reported in a cohort of patients hospitalized with influenza (8.0 percent and 8.8 percent, respectively). [Clin Infect Dis 2013;57:1069-1077]
Subtypes and seasonality
RSV is classified into two major subgroups, A and B. Both RSV A and RSV B typically cocirculate during the epidemic season, with year-to-year shifts in predominance. Generally, temperate countries in the Northern and Southern hemispheres experience peak RSV activity in winter. In tropical and subtropical countries and regions, such as Hong Kong, there is less seasonality and RSV is considered a year-round infection, albeit more common in the rainy season (April–August). [J Glob Health 2019;9:020431; Medicine 2015;94:e2024]
Bivalent RSV vaccine available in HK
A single-dose bivalent RSVpreF vaccine is now available in Hong Kong for prevention of LRTD caused by RSV in individuals ≥60 years of age, and for prevention of LRTD and severe LRTD caused by RSV in infants from birth to ≤6 months of age through active immunization of pregnant individuals at a gestational age of 32–36 weeks. [RSVpreF vaccine Hong Kong Prescribing Information, March 2024; https://www.pfizer.com.hk/en/news-en/pfizer%E2%80%99s-first-dual-indication-vaccine-for-respiratory-syncytial-virus-rsv-is-available-this-month-in-hong-kong/]
Efficacy and safety in older individuals
Indication of the RSVpreF vaccine in older individuals is supported by results of the ongoing phase III, multicentre, randomized, double-blind, placebo-controlled RENOIR trial. In this study initiated in August 2021, participants who were healthy or had stable chronic conditions (including chronic cardiopulmonary disease [eg, COPD, asthma]) were randomized 1:1 to receive RSVpreF vaccine 120 μg or placebo. The primary endpoints were vaccine efficacy against RSV-associated LRTD with ≥2 or ≥3 signs or symptoms (ie, cough, wheezing, sputum production, shortness of breath, or tachypnoea). [N Engl J Med 2023;388:1465-1477]
During the first complete RSV season for Northern and Southern hemispheres (August 2021–October 2022), vaccine efficacy (n=36,134) was 88.9 percent (95 percent CI, 53.6–98.7) against RSV-associated LRTD with ≥3 signs or symptoms, and 65.1 percent (95 percent CI, 35.9–82.0) against RSV-associated LRTD with ≥2 signs or symptoms. (Table) [N Engl J Med 2023;388:1465-1477; MMWR Morb Mortal Wkly Rep 2023;72:793-801]
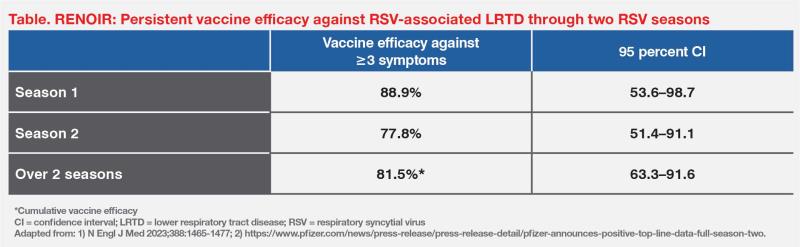
Results of the latest analysis (n=38,863) through the second complete RSV season showed sustained clinical benefit, with a vaccine efficacy of 77.8 percent (95 percent CI, 51.4–91.1) against RSV-associated LRTD with ≥3 signs or symptoms. The cumulative efficacy against RSV-associated LRTD with ≥3 signs or symptoms over the two seasons, spanning approximately 16.4 months of disease surveillance, was 81.5 percent (95 percent CI, 63.3–91.6). (Table) [Munjal I, ACIP 2024; https://www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-positive-top-line-data-full-season-two]
The RSVpreF vaccine was well tolerated in the RENOIR trial. Rates of adverse events (AEs) were similar between the RSVpreF vaccine and placebo groups through 1 month after injection (9.0 vs 8.5 percent; injection-related, 1.4 vs 1.0 percent). Injection-site pain was the most common local reaction (11.0 vs 6.0 percent), while fatigue (16.0 vs 14.0 percent) and headache (13.0 vs 12.0 percent) were the most common systemic events. Additionally, rates of severe or life-threatening AEs were similar between the RSVpreF vaccine and placebo groups (0.5 vs 0.4 percent), as were rates of serious AEs (2.3 vs 2.3 percent). [N Engl J Med 2023;388:1465-1477]
RSV vaccine recommendation
The US Centers for Disease Control and Prevention (CDC) has updated its single-dose RSV vaccination recommendation for adults to include all individuals ≥75 years of age and those 60–74 years of age at increased risk of severe RSV disease. [https://www.cdc.gov/vaccines/vpd/rsv/index.html]
According to experts in Hong Kong, available data support annual concomitant immunization with RSVpreF vaccine and seasonal inactivated influenza vaccine in older individuals. [Hong Kong Med J 2024;30:196-199; J Infect Dis 2022;225:2056-2066]
Conclusion
RSV can cause substantial morbidity and mortality among older individuals. A bivalent RSVpreF vaccine is now available in Hong Kong for prevention of RSV-associated LRTD in individuals ≥60 years of age. Clinical data showed that the single-dose vaccine was well tolerated and maintained consistently high protective efficacy through two full RSV seasons.