Contemporary management of CRSwNP with an anti–IL-5 biologic











Recurrence of nasal polyps despite standard-of-care (SoC) treatment is common in patients with chronic rhinosinusitis with nasal polyps (CRSwNP). At a recent symposium organized by the Hong Kong College of Otorhinolaryngologists, three Ear, Nose & Throat (ENT) specialists, namely, Professor Alkis Psaltis of The Queen Elizabeth Hospital, Adelaide, Australia, Dr Andrew Wong of Tuen Mun Hospital (TMH) and Dr Thomas Ho of Queen Mary Hospital (QMH), Hong Kong, shared insights into contemporary management of CRSwNP with surgery and an anti–interleukin (IL)-5 biologic (eg, mepolizumab), and discussed how a one-stop joint-specialty clinic approach optimizes management.
“Deeper understanding of chronic rhinosinusitis [CRS] has led to evolution of treatment from a focus on simple phenotypic manifestations [ie, polyps or no polyps] to individualized, integrated management pathways depending on the cause of disease and inflammation endotype [ie, type 2 vs non-type 2],” said Psaltis.
Surgery: More than ventilation
Initial SoC treatment of CRSwNP includes nasal irrigations, intranasal corticosteroids, oral antibiotics, short courses of oral corticosteroids (OCS), and nasal surgery. “Of note, surgery is not considered curative, but is rather a means to improve medical management,” said Psaltis. [Rhinology 2020;58:1-464; J Asthma Allergy 2021;14:873-882]
“The role of surgery in CRS treatment is not only to improve ventilation and drainage and reduce inflammatory burden, but also to facilitate access of topically applied drugs and assist in endotyping the disease process,” added Psaltis.
“During surgery, tissue samples from nasal polyps can be obtained for histopathological examination, which can reveal the type of inflammation [type 2 vs non–type 2], predominant inflammatory cell type [eg, eosinophils, neutrophils], inflammation severity, and presence of metaplasia or ulceration,” added Psaltis.
Joint clinic: A one-stop approach
CRSwNP is characterized by type 2 inflammation and often co-occurs with other type 2 inflammation–related conditions, including aspirin-exacerbated respiratory disease (AERD), asthma, and atopic dermatitis. [Heliyon 2023;9:e19249; ERJ 2020;56:232]
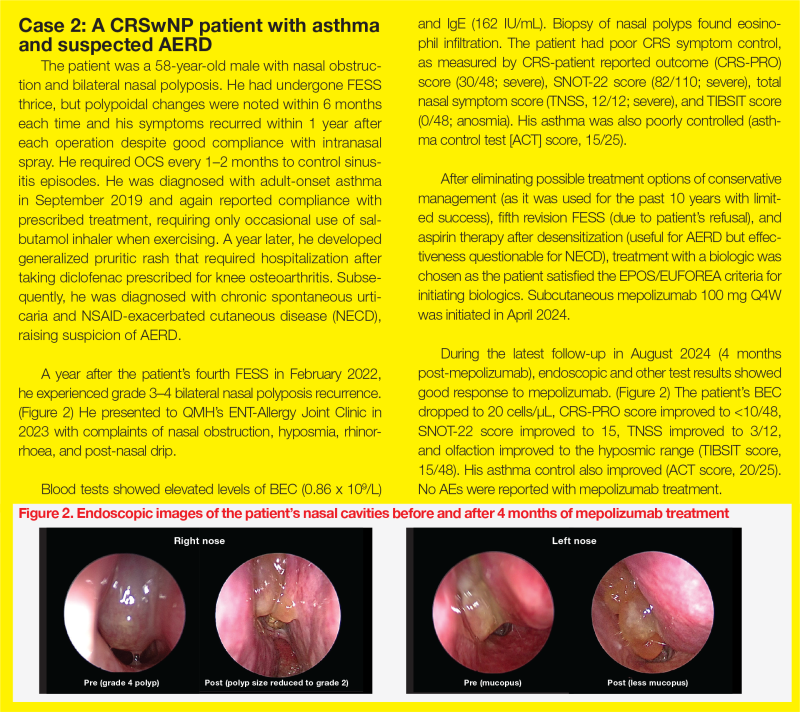
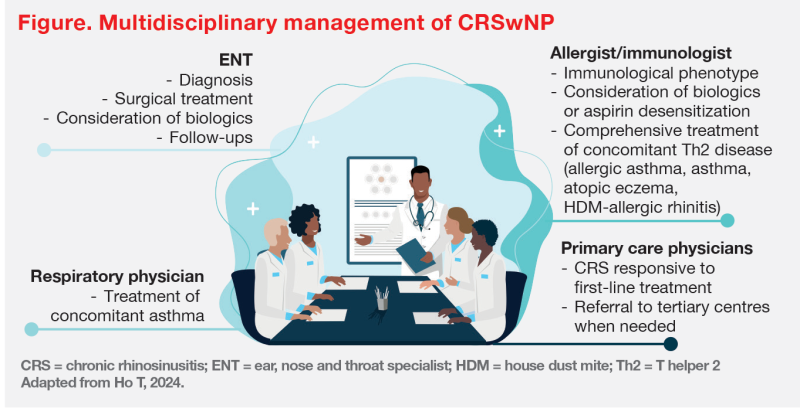
“[As illustrated in cases 1 and 2,] management of CRSwNP requires a multidisciplinary team effort,” Wong and Ho emphasized. (Figure)
TMH experience: Airway Combine Clinic
“Our monthly Airway Combine Clinic, which brings together ENT surgeons and respirologists, has been exclusively dedicated to obstructive sleep apnoea management for years,” said Wong. “In November 2023, we expanded our services to provide biologics [ie, mepolizumab and dupilumab] to eligible patients with CRSwNP.”
“In our clinic, we assess nasal polyp histology, conduct endoscopy, perform smell test [eg, top international biotech smell identification test (TIBSIT)], administer the Sino-nasal outcome test [SNOT]-22, measure immunoglobulin E [IgE] and eosinophil levels through blood tests, and manage comorbid asthma,” added Wong. “We coordinate biologic injections for eligible patients at the day ward and provide follow-up appointments to assess treatment compliance and monitor any adverse events [AEs].”
The eligibility criteria for biologics include a history of nasal surgery or being unfit for surgery, along with ≥3 of the following criteria, which are consistent with cases 1 and 2 and the European Position Paper on Rhinosinusitis and Nasal Polyps and European Forum for Research and Education in Allergy and Airway diseases (EPOS/EUFOERA) 2023 Guidelines
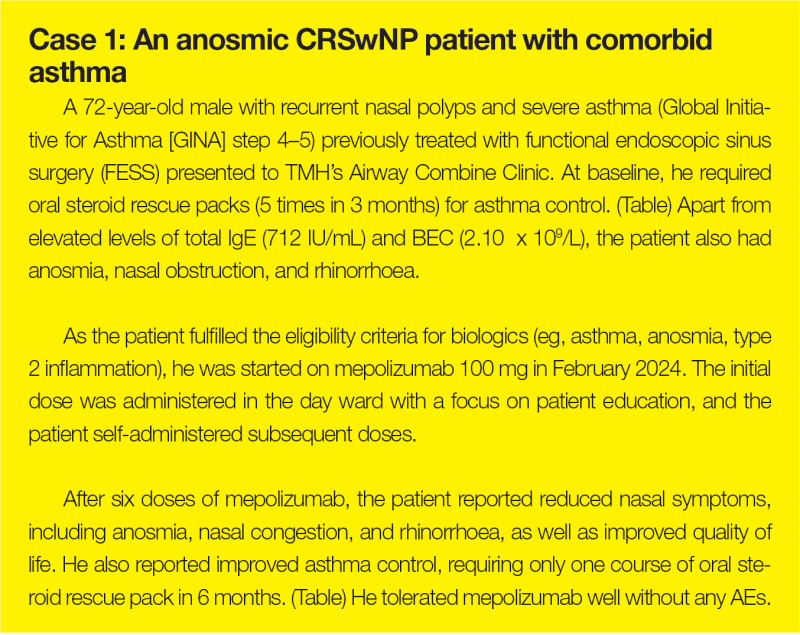
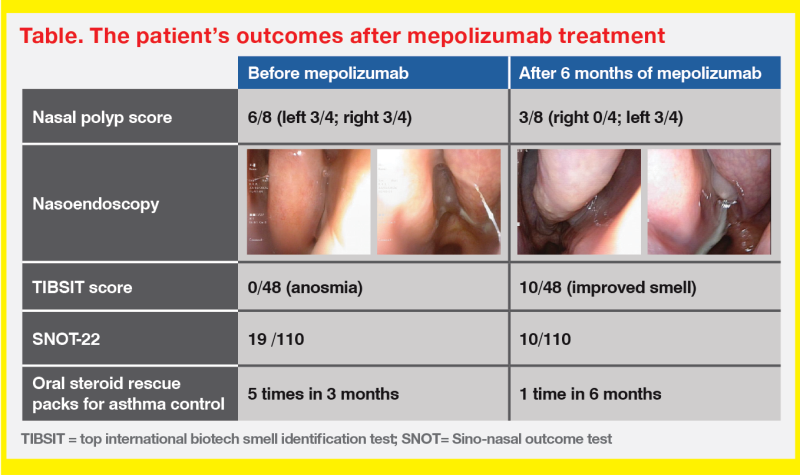
QMH experience: ENT-Allergy Joint Clinic
“A similar one-stop approach combining expertise in comprehensive diagnosis and treatment has been adopted at QMH,” said Ho. “The ENT and Allergy & Immunology Joint Clinic carries out nasal endoscopy, smell tests, aeroallergen skin prick tests, serum IgE screening, biologic screening and counselling, and initiation of sublingual immunotherapy.”
At the ENT-Allergy Joint Clinic, six patients have been started on mepolizumab for treatment of CRSwNP. “Most patients in our cohort achieved good response to mepolizumab in terms of nasal polyp score, nasal symptoms, and/or good asthmatic control,” reported Ho.
“Disease, patient, and treatment factors constitute key elements of personalized management,” said Ho. As illustrated in case 2, the presence of suspected AERD requires input from immunologists to optimize treatment.
Bridging trial data and local experience
Local experience, including cases 1 and 2, was generally consistent with findings of the phase III SYNAPSE trial, which included 407 patients with CRSwNP who were eligible for repeat nasal surgery despite SoC treatment and had ≥1 nasal surgery in the past 10 years. Patients were assigned to receive mepolizumab 100 mg subcutaneously or placebo Q4W, in addition to SoC. [Lancet Respir Med 2021;9:1141-1153]
“Results showed that adding mepolizumab to SoC significantly reduced endoscopic nasal polyp score at week 52 [adjusted difference in medians, -0.73; 95 percent confidence interval [CI] -1.11 to -0.34; p<0.0001] and nasal obstruction visual analogue score [VAS] in weeks 49– 52 [adjusted difference in medians, -3.14; 95 percent CI, -4.09 to -2.18; p<0.0001] vs placebo,” reported Psaltis.
Additionally, mepolizumab-treated patients had significantly improved nasal symptoms, including SNOT-22 total score at week 52 (adjusted difference in medians, -16.49; 95 percent CI, -23.57 to -9.42; p=0.0032) and loss of smell VAS symptom score during weeks 49–52 (adjusted difference in medians, -0.37; 95 percent CI, -0.65 to -0.08; p=0.020) vs placebo.