CROWN study at 5 years: Sustained benefits with lorlatinib in advanced ALK-positive NSCLC









The phase III CROWN trial previously showed that first-line treatment with lorlatinib, a third-generation ALK tyrosine kinase inhibitor (TKI), provided significant survival benefits vs crizotinib in patients with treatment-naïve, advanced, ALK-positive non-small-cell lung cancer (NSCLC). At the 2024 World Conference on Lung Cancer, CROWN investigators discussed the patterns of progression and management of adverse events (AEs) associated with lorlatinib after 5 years of follow-up.
mPFS not reached after 5 years of lorlatinib
The CROWN study is an ongoing global phase III trial comparing the efficacy and safety of lorlatinib vs crizotinib in 296 patients with untreated advanced ALK-positive NSCLC. After 5 years, lorlatinib continued to demonstrate significant survival benefits, with median progression-free survival (mPFS) not reached (NR), vs 9.1 months for crizotinib (hazard ratio [HR], 0.19; 95 percent confidence interval [CI], 0.13–0.27). The 5-year PFS rates were 60 percent for lorlatinib vs 8 percent for crizotinib. [N Engl J Med 2020;383:2018-2029; Solomon BJ, et al, ASCO 2024, abstract LBA8503]
Who is likely to progress sooner?
“Mean baseline tumour burden was greater [84.9 vs 54.7 mm] among lorlatinib-treated early progressors [≤12 months] vs patients who were progression-free after 5 years,” said Professor Tony Mok of the Chinese University of Hong Kong. [Mok T, et al, WCLC 2024, abstract MA06.07]
In addition, unconfirmed ALK positivity (50 percent in early progressors and 22 percent in nonprogressors) and confirmed TP53 mutations (57 percent in early progressors and 22 percent in nonprogressors) were more common among early progressors, while other clinical and molecular characteristics were similar between early progressors and nonprogressors.
PFS2 considerably longer with lorlatinib
“After 5 years of follow-up, [at the time of data cut-off (DCO), 31 October 2023] 50 percent of patients had discontinued lorlatinib, while 95 percent had discontinued crizotinib,” highlighted Mok. [Mok T, et al, WCLC 2024, abstract MA06.07]
Median PFS2, the time from randomization to progression on next-line therapy or death, was prolonged with lorlatinib vs crizotinib. After a median follow-up period of 61.4 months for the lorlatinib group and 58.4 months for the crizotinib group, median PFS2 was NR vs 37.9 months for lorlatinib and crizotinib, respectively (HR, 0.43; 95 percent CI, 0.30–0.62). The respective 5-year PFS2 rates were 67 vs 37 percent. (Figure)
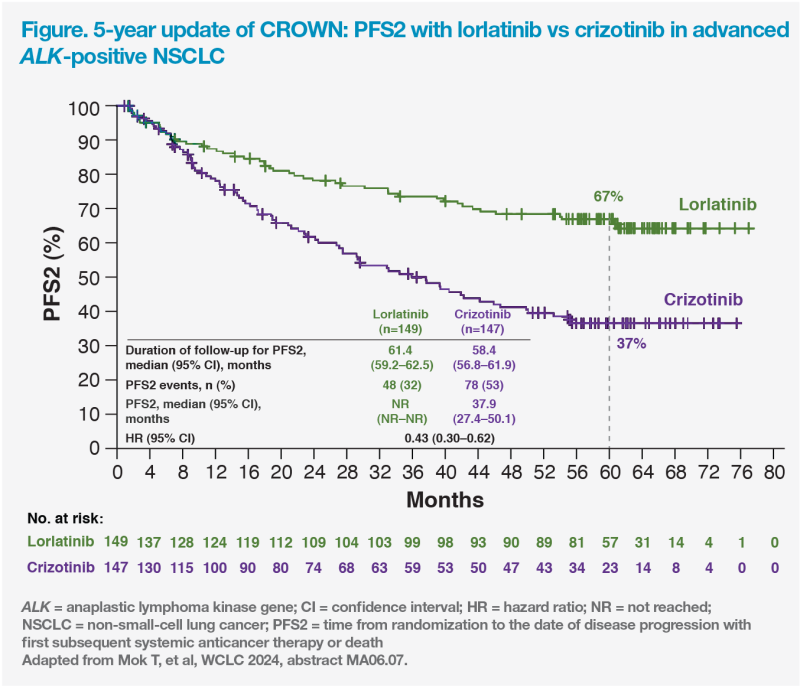
“This prolonged PFS2 gives us an insight on what the overall survival data may be like, which I anticipate will become available after my retirement,” quipped Mok.
Subsequent anticancer therapy
At 5 years, at DCO, 38 patients in the lorlatinib group and 109 in the crizotinib group received ≥1 subsequent systemic anticancer therapy. ALK TKIs were the first subsequent treatment in 61 and 93 percent of patients, respectively, with chemotherapy being the first subsequent treatment in 39 and 4 percent, respectively.
Median duration of the first subsequent treatment (DOT) was 9.3 months in the lorlatinib group and 14.9 months in the crizotinib group. For those who received ALK TKIs as first subsequent therapy, the median DOT was similar between the two groups, at 12.5 and 15.8 months, respectively, suggesting that this strategy may be a valid subsequent therapy option for either treatment groups. [Mok T, et al, WCLC 2024, abstract MA06.07 presentation]
Importantly, overall objective response rate with the first subsequent systemic anticancer therapy was higher in patients previously treated with lorlatinib vs crizotinib (23.7 vs 17.4 percent). This suggests that subsequent systemic treatments may offer greater benefit in patients who had received lorlatinib.
Lorlatinib’s safety after 5 years of follow-up
“Post hoc analysis of the CROWN study indicated no new safety signals associated with long-term lorlatinib treatment,” said Dr Todd Bauer of the Greco-Hainsworth Centers in Nashville, US. [Bauer T, et al, WCLC 2024, abstract MA06.08]
Treatment discontinuation remained low, with all-causality AEs leading to permanent discontinuation in 11 percent of patients in the lorlatinib arm. [J Clin Oncol 2024;doi:10.1200/JCO.24.00581]
In patients who continued with lorlatinib, AEs were efficiently managed with active interventions, including dose modifications. In patients who required at least one lorlatinib dose reduction (33 percent), median time to first dose reduction was 21.6 weeks. “Importantly, dose reduction did not seem to impact median PFS or time to intracranial progression,” noted Bauer. [Bauer T, et al, WCLC 2024, abstract MA06.08; J Clin Oncol 2024;doi:10.1200/ JCO.24.00581]
Time to onset and duration of AEs
Most AEs in CROWN occurred within the first 4 months of treatment, with grade ≥3 events occurring within the first 9 months. [Bauer T, et al, WCLC 2024, abstract MA06.08]
Hypercholesterolaemia of any grade occurred in 108 of 149 patients (72 percent) in the lorlatinib group. Median time to onset of any-grade event was 15 days, while median duration was approximately 37 months. These were generally managed with lipid-lowering agents. “Pitavastatin, pravastatin, or rosuvastatin should be considered upfront due to their low involvement with CYP3A4,” advised Bauer. [Bauer T, et al, WCLC 2024, abstract MA06.08 presentation; Clin Pharmacol Ther 2006;80:565-581]
Central nervous system (CNS) effects had a median time to onset of 116 days and median duration of approximately 8 months. [Bauer T, et al, WCLC 2024, abstract MA06.08] CNS AEs, which occurred in 42 percent of patients, mostly were of grade 1/2, did not increase in prevalence or incidence over time, and did not require any medical intervention. [Bauer T, et al, WCLC 2024, abstract MA06.08 presentation]
Weight gain occurred in 44 percent of patients in the lorlatinib group. Most patients (95 percent) did not require medical intervention for their weight gain, which was primarily managed with lifestyle modifications. Lorlatinib dose reduction, interruption or both were required in the remaining patients. Weight gain resolved with no medical intervention in 35 percent of patients.
“Weight gain and oedema appeared to correlate only in 40 percent of patients, suggesting different pathogenic mechanisms,” remarked Bauer.
Summary
The 5-year follow-up of the CROWN trial supports the durable efficacy of lorlatinib vs crizotinib, even after progression on first-line therapy. Importantly, no new safety signals emerged with long-term lorlatinib treatment, and most AEs resolved with dose modifications, which did not appear to impact PFS.