Early initiation of extended adjuvant therapy in a high-risk patient with early-stage HER2-positive breast cancer





Presentation and investigations
A 35-year-old premenopausal female who was otherwise healthy presented in July 2022 with a mass in the left breast and palpable lymph nodes (LN) in the left axillary area. She was asymptomatic, and her Eastern Cooperative Oncology Group (ECOG) performance status (PS) score was 0–1.
Investigations revealed a tumour measuring 3.1 x 2.1 x 4.9 cm in the upper outer quadrant of the left breast and multiple enlarged LNs, suggesting metastasis. Biopsy showed ductal carcinoma in situ with focal stromal invasion in left breast, and metastasis was detected in upper and lower LNs of the left clavicle. Immunohistochemical analysis indicated that the tumour was HER2-positive, oestrogen receptor (ER)–negative and progesterone receptor (PR)–negative, with a Cerb-B2 score of 3+ and a Ki-67 level of approximately 40 percent. The patient was diagnosed with breast carcinoma (BC; cT3N3M0, stage IIIc, HER2 overexpression type).
Treatment
The patient commenced neoadjuvant docetaxel with carboplatin plus dual HER2 blockade (trastuzumab and pertuzumab) in July 2022. After six cycles, partial response was achieved. In November 2022, the patient underwent modified radical mastectomy. Postoperative pathology showed residual disease, and metastasis was detected in one of the LNs. Immunohistochemistry showed invasive carcinoma, ductal carcinoma in situ and metastatic carcinoma in LNs. Pathological complete response (pCR) was not achieved.
Subsequently, the patient underwent chest wall and supraclavicular radiotherapy in December 2022 and received adjuvant therapy with 14 cycles of trastuzumab emtansine (T‑DM1) until October 2023. In November 2023, she started extended adjuvant therapy with neratinib in a dose-escalating manner: 120 mg daily in week 1, 160 mg daily in week 2 and 240 mg daily from week 3 onwards. During the first month of treatment, she experienced grade 2 diarrhoea (4–5 times/day), which improved with loperamide PRN. As of August 2024, the patient has been on neratinib for 6 months, and her general condition remains good.
Discussion
HER2-positive BC is associated with an increased recurrence risk and worse prognosis vs HER2-negative BC. Although the addition of trastuzumab to adjuvant chemotherapy has been shown to extend overall survival (OS) by nearly one-third in patients with HER2-positive BC, up to 31 percent of them experience recurrence and/or die within 10 years of treatment.1-3
Even with optimal (neo)adjuvant therapy, distant recurrences are still observed with longer follow-up. Typically, patients who do not achieve pCR after neoadjuvant therapy and have larger tumour size (≥2 cm) or nodal involvement are at high recurrence risk.3 Younger patients with higher recurrence risk characteristics, including higher histological grading, Ki-67 expression and proportion of vascular invasion, have worse prognosis than older patients.4
The median age of BC onset in China and East Asian countries (45–49 years) is >10 years lower than in Western countries (62–64 years), and early BC (eBC) cases are more prevalent in younger adults in mainland China(<40 years of age, 14.9 percent of all incident cases; <35 years of age, 6.5 percent) and Hong Kong (<44 years of age, 13.9 percent) vs Western countries (<40 years of age, 4.9 percent).4,5
As our young patient was considered at high risk of recurrence, she received guideline-recommended adjuvant treatment with T-DM1.2,6 Adjuvant T-DM1 demonstrated a 50 percent reduction in risk of recurrence or death vs trastuzumab in the phase III KATHERINE study. However, the risk of recurrence with adjuvant T-DM1 remained substantial, with 11.7 percent of patients relapsing at 3 years.7 Although T-DM1 reduced the risk of distant recurrence as the first event by 40 percent vs trastuzumab, a subset of these patients experienced central nervous system (CNS) recurrence as their first event: 5.9 percent in the T-DM1 group and 4.3 percent in the trastuzumab group. These results suggest that T-DM1 provides good control of visceral disease, while highlighting an unmet medical need for effective post-neoadjuvant treatment to prevent recurrence in the CNS.7-9
Neratinib is an oral, small-molecule irreversible pan-HER tyrosine kinase inhibitor (TKI) indicated in adults with HER2-positive eBC who have completed adjuvant trastuzumab-based therapy ≤1 year ago. In the phase III ExteNET trial (n=2,840), patients with HER2-positive eBC who initiated neratinib ≤1 year after trastuzumab-based therapy demonstrated an absolute 5-year invasive disease-free survival (iDFS) benefit of 5.1 percent (hazard ratio [HR], 0.58; 95 percent confidence interval [CI], 0.41–0.82) and an absolute 8-year OS benefit of 2.1 percent (HR, 0.79; 95 percent CI, 0.55–1.13) vs placebo. Among patients with residual disease after neoadjuvant treatment (non-pCR subgroup; n=295), absolute 5-year iDFS and 8-year OS gains with neratinib were 7.4 percent (HR, 0.60; 95 percent CI, 0.33–1.07) and 9.1 percent (HR, 0.47; 95 percent CI, 0.23–0.92), respectively.10
Neratinib may pass through the blood-brain barrier (BBB) by inhibiting ATP-binding cassette B1 transport function, which is one of the dominant efflux transporters in the BBB, which may explain its efficacy in minimizing CNS recurrence.11 ExteNET demonstrated a lower 5-year cumulative incidence of first CNS recurrences with neratinib vs placebo (0.7 vs 2.1 percent), and more patients in the neratinib group were alive without a CNS recurrence at 5 years (98.4 vs 95.7 percent; HR for CNS-DFS, 0.41; 95 percent CI, 0.18–0.85). The potential CNS benefit was consistently shown across various high-risk subgroups, including non-pCR and node-positive patients.10 (Table)
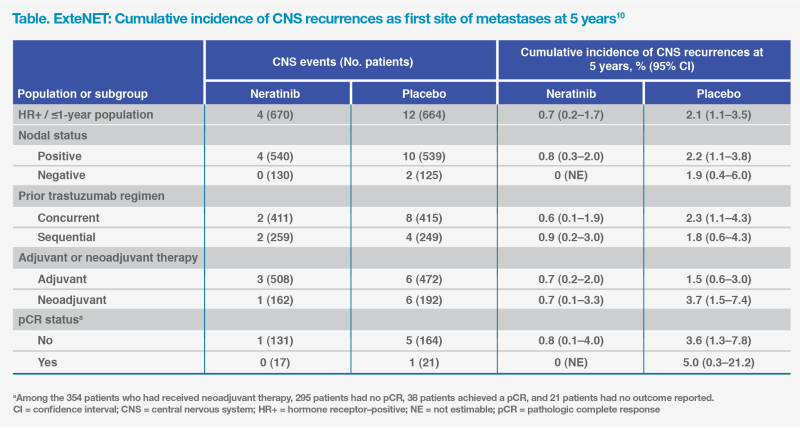
In summary, extended adjuvant therapy with neratinib may prolong iDFS in patients with HER2-positive eBC at high risk of recurrence. This case illustrates the feasibility of early initiation of extended adjuvant neratinib and the effectiveness of proactive antidiarrhoeal measures.