
Elranatamab monotherapy demonstrated a median progression-free survival (mPFS) of 17.2 months and median overall survival (mOS) of >2 years in heavily pretreated patients with relapsed or refractory multiple myeloma (R/R MM) who had received a median of five prior lines of therapy, with no new safety signals, according to long-term follow-up data from the MagnetisMM-3 trial presented at the 29th Congress of the European Hematology Association (EHA 2024).
Dual-target bsAb: BCMA x CD3
Patients with R/R MM often have limited therapeutic options as their disease progresses, resulting in increasingly shorter remission with every treatment line. [Blood Rev 2021:49:100808]
Elranatamab is a bispecific antibody (bsAb) targeting B-cell maturation antigen (BCMA) and CD3. It is indicated for treatment of R/R MM in patients who have received ≥3 prior therapies, including an immunomodulatory drug (IMiD), a proteasome inhibitor (PI), and an anti-CD38 antibody, and have demonstrated disease progression on the last therapy. [Nat Med 2023;29:2259-2267; Elrexfio Hong Kong Prescribing Information, March 2024]
MagnetisMM-3: Elranatamab in BCMAnaïve R/R MM
The open-label, single-arm, multicentre, phase II MagnetisMM-3 trial evaluated the efficacy and safety of elranatamab monotherapy in 123 patients with R/R MM (median age, 68.0 years; male, 55.3 percent) who had not received prior BCMA-directed therapy. [Nat Med 2023;29:2259-2267]
Heavily pretreated population
At baseline, a high proportion of patients (76.4 percent) had ≥1 poor prognostic features, including pentarefractory disease (ie, refractory to ≥2 PIs, ≥2 IMiDs and ≥1 anti-CD38 antibody; 42.3 percent), extramedullary disease (assessed by blinded independent central review [BICR]; 31.7 percent), high-risk cytogenetics (ie, t[4;14], t[14;16] or del[17p] chromosomal abnormalities; 25.2 percent), bone marrow plasma cells ≥50 percent (21.1 percent), stage III disease based on the Revised International Staging System (15.4 percent), and Eastern Cooperative Oncology Group performance status of 2 (5.7 percent).
Most patients in MagnetisMM-3 were heavily pretreated:
- Median prior lines of therapy, 5 (range, 2–22)
- Triple-class exposure, 100 percent
- Triple-class refractory disease, 96.7 percent
- Refractory to the last line of therapy, 95.9 percent
Deep and durable responses
Elranatamab monotherapy achieved the primary endpoint of objective response rate (ORR) as assessed by BICR at the previous data cut-off (median follow-up, 14.7 months) of the MagnetisMM-3 trial. [Nat Med 2023;29:2259-2267]
After a median follow-up of 28.4 months by reverse Kaplan-Meier method (data cut-off, 26 March 2024), elranatamab continued to demonstrate deep and durable responses in BCMA-naive patients with R/R MM. [Mohty M, et al, EHA 2024, poster P932]
ORR was high at 61.0 percent in this heavily pretreated population, despite the high proportion of patients with poor prognostic features at baseline. More than one-third (37.4 percent) of patients achieved complete response (CR) or better, with 21.1 percent achieving CR and 16.3 percent achieving stringent CR. Among evaluable patients (those with ≥CR evaluable for minimal residual disease [MRD]; n=31), 90.3 percent achieved MRD negativity at a threshold of 10-5.
Median duration of response (DoR) was not reached in patients with ≥CR or objective response. The probability of maintaining a response at 2 years was 87.9 percent (95 percent confidence interval [CI], 73.1–94.8) in patients with ≥CR and 66.9 percent (95 percent CI, 54.4–76.7) in those with objective response. (Figure 1)
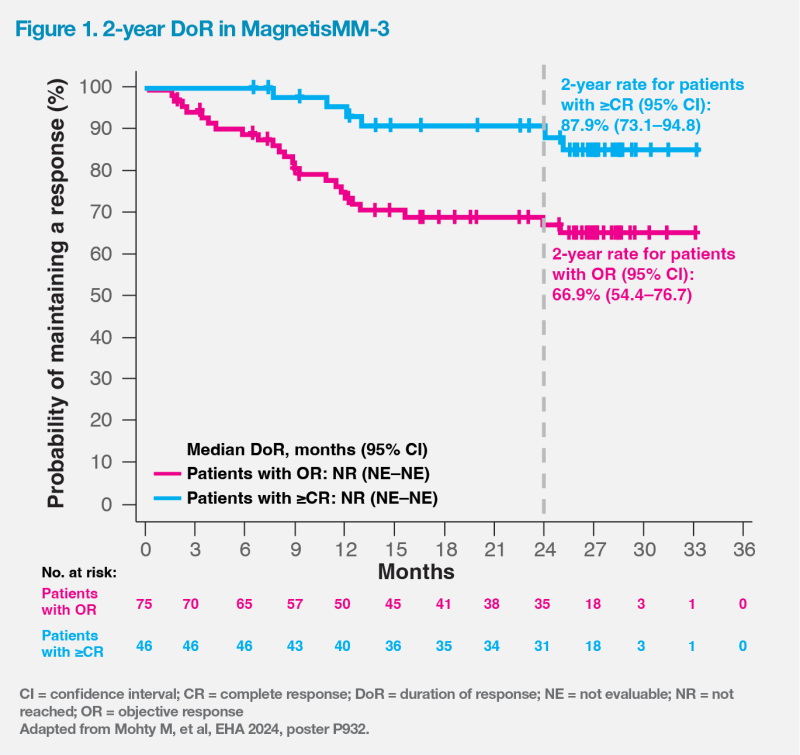
Longest PFS among anti- BCMA bsAbs
Notably, long-term follow-up results of MagnetisMM-3 demonstrated the longest reported mPFS among anti-BCMA bsAbs. [https://www.pfizer. com/news/press-release/press-release-detail/elrexfiotm-shows-median-overall-survival-more-two-years]
mPFS was 17.2 months (95 percent CI, 9.8–not evaluable [NE]), and mOS was 24.6 months (95 percent CI, 13.4–NE). These were markedly longer than those observed in triple class–refractory patients in the real-world LocoMMotion study, which reported a mPFS of 4.6 months and a mOS of 12.4 months. [Leukemia 2022;36:1371-1376; Mohty M, et al, EHA 2024, poster P932] In patients with ≥CR, mPFS was not reached, and the 2-year PFS rate was 90.6 percent (95 percent CI, 76.9–96.4). (Figure 2)
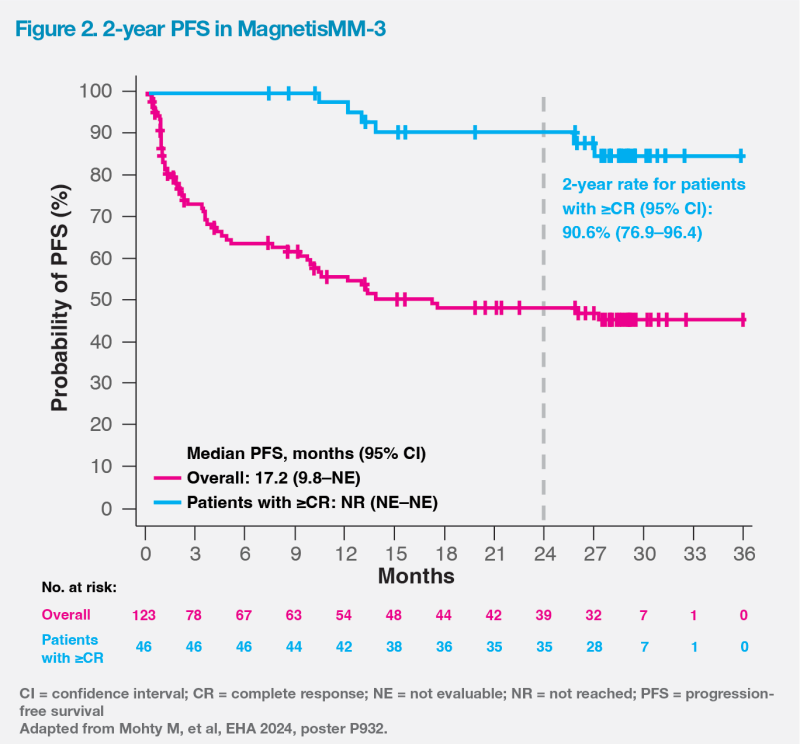
Manageable safety profile
The most common treatmentemergent adverse events (AEs) reported in MagnetisMM-3 were infections (any grade, 69.9 percent; grade 3–4, 39.8 percent), cytokine release syndrome (CRS; any grade, 57.7 percent; grade 3–4, 0 percent), and haematologic-related AEs, including anaemia (any grade, 48.8 percent; grade 3–4, 37.4 percent) and neutropenia (any grade, 48.8 percent; grade 3–4, 48.8 percent). Haematologic-related AEs were generally manageable with dose reductions or interruptions, and supportive therapies. Immune effector cell–associated neurotoxicity syndrome (ICANS) was infrequent (3.4 percent) and of grade 1–2. [Nat Med 2023;29:2259-2267]
No new safety signals were observed in the long-term follow-up. Five patients (4.1 percent) experienced secondary primary malignancies (SPMs), all of which were squamous-cell carcinomas of the skin, and all had previously received treatment with lenalidomide and stem-cell transplant. No haematologic SPMs were observed. [Mohty M, et al, EHA 2024, poster P932]
Biweekly dosing
Patients received subcutaneous elranatamab in two step-up doses (12 mg on day 1 and 32 mg on day 4), followed by a full treatment dose of 76 mg QW. After ≥24 weeks of treatment, persistent responders could switch to a Q2W dosing schedule. Importantly, the incidence of grade 3–4 AEs with Q2W dosing dropped from 58.6 to 46.6 percent without compromising efficacy. [Elrexfio Hong Kong Prescribing Information, March 2024; Nat Med 2023;29:2259-2267]