Gilteritinib improves survival in R/R FLT3-mutated AML





Patients with relapsed/refractory (R/R) acute myeloid leukaemia (AML) often have poor prognosis and limited succeeding treatment options. In particular, the presence of FMS-like tyrosine kinase 3 (FLT3) gene mutation adversely affects survival outcomes. At an industry-sponsored symposium, Professor Mark Levis from the Johns Hopkins University in Baltimore, US, discussed the role of the oral selective FLT3 inhibitor, gilteritinib, in patients with R/R FLT3-mutated AML, and shared case studies to demonstrate its applicability in select patients in his clinical practice.
Management considerations in R/R AML
“Patients with R/R AML have poor prognosis with salvage chemotherapy,” noted Levis. “An international randomized phase III study demonstrated that commonly used salvage regimens did not provide clinically meaningful benefit for those with R/R AML.” [J Clin Oncol 2014;32:1919-1926]
“Molecular profiling is important for patients with AML since the majority [86 percent] may have ≥2 driver mutations,” he explained. “The general pattern of AML pathogenesis involves a founder mutation, a cooperating mutation to generate the malignant founding clone, and a signalling mutation that contributes to disease progression. Characterizing these mutations may help inform disease classification and prognostic stratification, leading to a more personalized treatment approach.” [N Engl J Med 2016;374:2209-2221; Cell 2012;150:264-278]
FLT3-mutated AML
One of the signalling mutations in AML is FLT3. FLT3 is a cytokine receptor tyrosine kinase expressed in early haematopoietic stem and progenitor cells and is responsible for regulating their proliferation and differentiation. [Cell 2012;150:264-278; Proc Natl Acad Sci USA 1994;91:459- 463] FLT3-activating mutations are present in approximately 30 percent of patients with AML, usually as inframe internal tandem duplications (ITD) or as missense point mutations in the tyrosine kinase domain (TKD). [Leuk Res 2010;34:752-756; Blood 2022;140:1345-1377]
FLT3-ITD mutations in AML are traditionally known to adversely affect survival. [N Engl J Med 2016;374:2209-2221; Blood 1999;93:3074-3080; Blood 2001;98:1752-1759; Blood 2002;100: 4372-4380; Blood 2017;129:565-571] These mutations are also shown to increase the risk of relapse in general and after haematopoietic stem cell transplantation (HSCT). [Blood 2002;99:4326-4335; Cancer 2016;122:3005-3014]
Detecting FLT3-ITD mutations
“Polymerase chain reaction [PCR], the usual method for detecting FLT3- ITD mutations, is limited by its poor sensitivity [ie, approximately 1 percent] due to template bias,” explained Levis. [Blood 2002;99:4326-4335; Leuk Res Rep 2020;13:100198; Appl Environ Microbiol 1998;64:3724-3730; Blood Adv 2018;2:825-831]
Detection may also be compromised by mutational changes in patients’ AML. For instance, FLT3 mutations may not emerge until relapse and may therefore be undetectable at diagnosis, as demonstrated in case 1. [Blood 2002;100:2387-2392; Blood 2021;137:3093-3104]
Meanwhile, a highly sensitive combination PCR–next-generation sequencing (NGS) measurable residual disease (MRD) assay for FLT3–ITD mutations has been developed and validated, which may improve sensitivity in detecting FLT3-ITD mutations. [Blood 2020;135:75-78; Blood Adv 2018;2:825-831]
R/R FLT3-mutated AML: Why gilteritinib?
Over the last two decades, FLT3 inhibitors have markedly improved survival outcomes vs chemotherapy or placebo in those with newly diagnosed or R/R FLT3-mutated AML. [Blood 2017;129:565-571; N Engl J Med 2019;381:1728-1740; Transplant Cell Ther 2023;29:265.e1-265.e10; N Engl J Med 2017;377:454-464; Lancet Oncol 2020;21:1201-1212; Lancet 2023;401:1571-1583]
In particular, single-agent gilteritinib, a potent and selective oral type I FLT3 inhibitor, is shown to improve survival of patients with R/R FLT3-mutated AML vs salvage chemotherapy. [Blood Adv 2020;4:1178-1191]
ADMIRAL trial: Improved OS with gilteritinib monotherapy
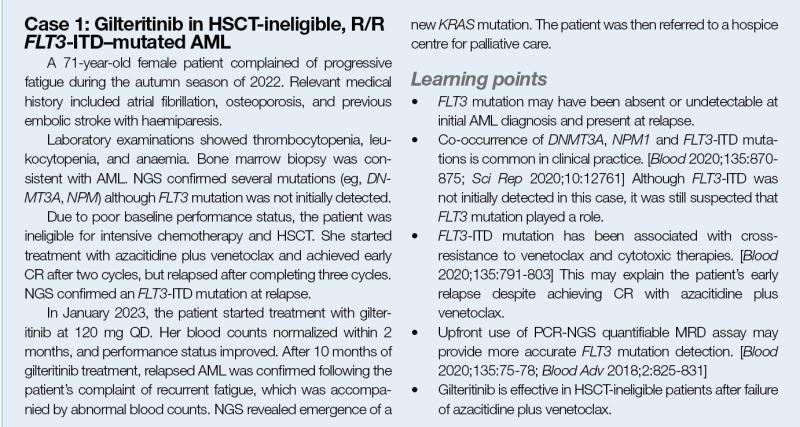
Gilteritinib monotherapy was approved for R/R FLT3–mutated AML based on the phase III ADMIRAL trial, which showed improved outcomes with gilteritinib monotherapy vs investigator-choice salvage chemotherapy. [N Engl J Med 2019;381:1728-1740]
In ADMIRAL, patients with R/R FLT3-mutated AML were randomized 2:1 to receive either gilteritinib 120 mg QD or salvage chemotherapy. The two primary endpoints were overall survival (OS) and the percentage of patients who had complete remission (CR) with full or partial haematologic recovery (CR/CRh).
Gilteritinib significantly extended median OS (9.3 vs 5.6 months; hazard ratio [HR], 0.64; 95 percent confidence interval [CI], 0.49–0.83; p<0.001) vs salvage chemotherapy. (Figure 1)
CR/CRh was achieved in a higher percentage of patients in the gilteritinib vs salvage chemotherapy group (34.0 vs 15.3 percent; risk difference, 18.6 percentage points; 95 percent CI, 9.8–27.4). “Gilteritinib was also associated with a median duration of CR/CRh of 11.0 months, [which was consistent with case 1],” said Levis.
Of the 197 patients in the gilteritinib group who were transfusion-dependent at randomization, 68 (34.5 percent) became transfusion-independent, which was consistent with cases 1 and 2, who achieved normalization of haemoglobin levels and platelet counts following gilteritinib treatment.
Gilteritinib was well tolerated in ADMIRAL, with grade ≥3 adverse events (AEs) and serious AEs occurring less frequently vs the chemotherapy group. The most common grade ≥3 AE in the gilteritinib group was myelosuppression (ie, febrile neutropenia; 45.9 percent), anaemia (40.7 percent) and thrombocytopaenia (22.8 percent).
Gilteritinib: Active against FLT3-TKD and -ITD mutations
Gilteritinib binds at the adenosine triphosphate (ATP) site. As its binding is relatively less influenced by the conformation of the activation loop, it is active against both FLT3-ITD and FLT3-TKD mutations. [Oncotarget 2019;10:2530-2545; Blood Adv 2020;4:1178-1191]
In contrast, type II FLT3 inhibitors bind to a hydrophobic pocket adjacent to the ATP site. The pocket is not accessible unless the activation loop of the kinase is in active conformation. “Since quizartinib is a selective type II FLT3 inhibitor, it is only effective against FLT3-ITD mutations, resulting in rapid emergence of FLT3- TKD mutations when used as monotherapy in patients with relapsed FLT3-ITD–mutated AML,” said Levis. [Blood Adv 2020;4:1178-1191]
“The emergence of FLT3-TKD mutations as a resistance mechanism appears specific to type II FLT3 inhibitors,” noted Levis. [Blood Adv 2020;4:1178-1191] “For instance, a cohort study involving patients with R/R FLT3-ITD–mutated AML showed that response to the type II FLT3 inhibitor, sorafenib, was lost after a median of approximately 10 weeks [72 days] with the emergence of FLT3-TKD mutations.” [Blood 2012;119:5133-5143]
Doubled OS with continued gilteritinib after HSCT
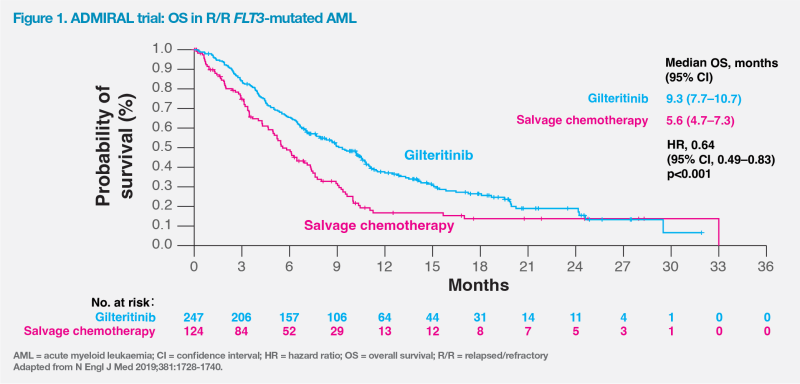
Final results of ADMIRAL continued to demonstrate survival benefits (median OS, 9.3 vs 5.6 months; HR, 0.637; 95 percent CI, 0.49–0.83; p=0.0007; 1-year OS rate, 37.1 vs 16.7 percent) and higher combined CR/CRh (34.0 vs 15.3 percent; p=0.0001) and CR rates (21.1 vs 10.5 percent; p=0.0106) with gilteritinib vs salvage chemotherapy. [Perl AE, et al, AACR 2019, abstract CT184]
“When feasible, HSCT should be offered to most patients with FLT3-ITD–mutated AML in CR,” said Levis. [Blood 2022;140:1345-1377] “After HSCT, patients in ADMIRAL who received gilteritinib maintenance had significantly longer median survival [16.2 vs 8.4 months; HR, 0.387; 95 percent CI, 0.164–0.915; p=0.024] vs those who did not resume gilteritinib. This suggests that bridging to transplant and post-transplant maintenance therapy with gilteritinib may be a promising therapeutic option for R/R FLT3-mutated AML to improve treatment outcomes, as illustrated in case 2.” (Figure 2) [Perl AE, et al, AACR 2019, abstract CT184; Case Rep Hematol 2023;2023:7164742]
“There is clinical and laboratory evidence demonstrating the antileukaemic synergy between FLT3 inhibition and allogeneic immune effects to induce durable remissions,” noted Levis. [Leukemia 2012;26:2353- 2359; Nat Med 2018;24:282-291]

Summary
The treatment landscape for patients with R/R AML has markedly changed with the advent of personalized medicine and the emergence of newer agents targeting specific driver mutations. In particular, monotherapy with the type I FLT3 inhibitor, gilteritinib, has demonstrated superior OS vs salvage chemotherapy in patients with R/R FLT3-mutant AML, including HSCT-eligible (as bridging and post-HSCT maintenance) and HSCT-ineligible patients.