GLP-1 RAs offer CV benefits in T2DM





Cardiovascular (CV) benefits of glucagon-like peptide 1 receptor agonists (GLP-1 RAs) support their use in type 2 diabetes mellitus (T2DM). At a Novo Nordisk–sponsored lecture during the 6th Annual Meeting of Endocrinology, Diabetes and Metabolism Hong Kong (EDM HK), Dr Julie Lovshin of the Division of Endocrinology and Metabolism, University of Toronto, Canada, reviewed clinical trials and real-world evidence on CV benefits provided by GLP-1 RAs, such as semaglutide, which led to guideline recommendations on their use in T2DM. Safety concerns related to GLP-1 RAs were also addressed.
GLP-1 RAs: Cardioprotective effects in T2DM
GLP-1 RAs’ cardioprotective effects in patients with T2DM were established in CV outcome trials (CVOTs). A meta-analysis of eight randomized placebo-controlled CVOTs involving 60,080 T2DM patients demonstrated a 14 percent relative risk reduction (RRR) in three-point major adverse CV events (MACE; ie, stroke, myocardial infarction [MI], CV death) with use of GLP-1 RAs (hazard ratio [HR], 0.86; 95 percent confidence interval [CI], 0.80–0.93; p<0.0001), with a number needed to treat (NNT) of 65 over a weighted average median follow-up of 3.0 years. Furthermore, GLP-1 RAs were associated with significantly reduced risks of individual MACE components, namely, fatal or nonfatal stroke (HR, 0.83; 95 percent CI, 0.76–0.92; p=0.0002), fatal or nonfatal MI (HR, 0.90; 95 percent CI, 0.83–0.98; p=0.020), and CV death (HR 0.87; 95 percent CI, 0.80–0.94; p=0.0010). [Lancet Diabetes Endocrinol 2021;9:653-662]
SUSTAIN-6 trial of semaglutide
SUSTAIN-6 was an event-driven, noninferiority CVOT, which evaluated efficacy of subcutaneous (SC) semaglutide (0.5 mg Q1W or 1.0 mg Q1W) vs placebo in addition to standard care in patients with T2DM, including those with established CV disease (CVD), chronic heart failure, or stage ≥3 chronic kidney disease (CKD) at ≥50 years of age, and those with ≥1 CV risk factor at ≥60 years of age. [N Engl J Med 2016;375:1834-1844; Diabetes Obes Metab 2020;22:442-451]
A total of 3,297 patients (mean age, 64.6 years; male, 60.7 percent; mean duration of T2DM, 13.9 years) were randomized 1:1 to receive semaglutide or placebo, of whom 3,232 (98.0 percent) completed the trial. Most patients (83.0 percent) had established CVD and/or ≥stage 3 CKD. The most commonly used glucose-lowering medications were metformin (73.2 percent) and insulin (58.0 percent).
With a median follow-up of 2.1 years, the primary composite outcome of first nonfatal stroke, nonfatal MI or CV death occurred in 6.6 percent vs 8.9 percent of patients in the semaglutide vs placebo group, confirming noninferiority of semaglutide vs placebo (HR, 0.74; 95 percent CI, 0.58–0.95; pnoninferiority<0.001). A 26 percent RRR in the primary outcome was achieved with semaglutide vs placebo. (Figure 1) The NNT was 45 over 2 years.
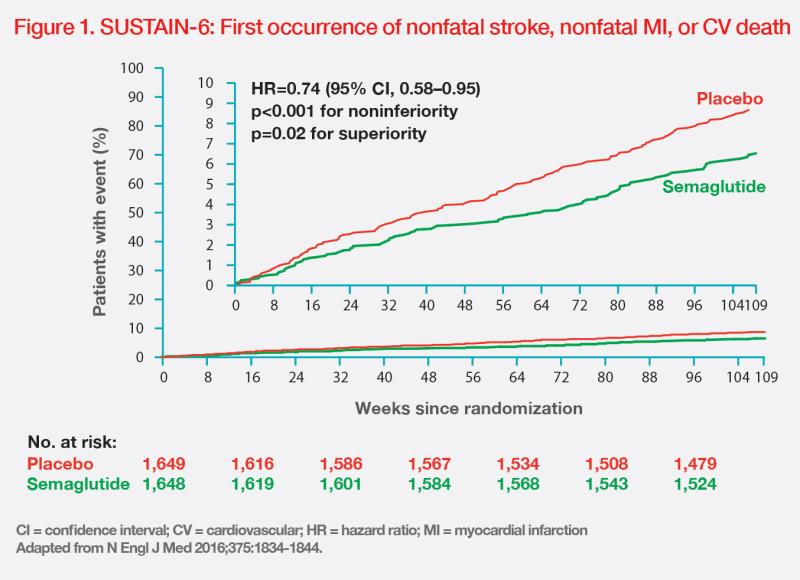
“The separation of event curves after 12–18 months suggests that GLP-1 RAs’beneficial effects are mediated by a reduction in atherosclerotic events. With antiinflammatory properties, GLP-1 RAs gradually reduce [development and progression of] atherosclerotic lesion,” noted Lovshin. (Figure 1) [N Engl J Med 2016;375:1834-1844; Circulation 2022;146:1882-1894] Mechanisms of cardiovascular effects of sodiumglucose cotransporter 2 inhibitors include early natriuresis and reduced inflammation and pro-inflammatory cytokine production. [Nat Rev Cardiol 2020; 17:761-772]
The reduction in risk of primary composite outcome with semaglutide was mainly driven by a significant 39 percent RRR in nonfatal stroke (HR, 0.61; 95 percent CI, 0.38–0.99; p=0.04). (Figure 2) [N Engl J Med 2016;375:1834-1844]
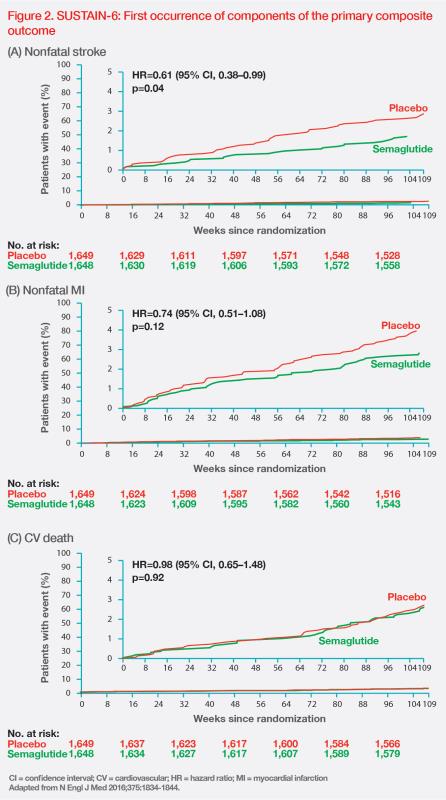
Semaglutide was also associated with a 26 percent RRR in the expanded composite outcome of nonfatal stroke, nonfatal MI, CV death, revascularization, and hospitalization for unstable angina or heart failure (HR, 0.74; 95 percent CI, 0.62–0.89; p=0.002) vs placebo.
The risk of new or worsening nephropathy was lower with semaglutide vs placebo (HR, 0.64; 95 percent CI, 0.46–0.88; p=0.005), but the risk of diabetic retinopathy complications was higher with semaglutide (HR, 1.76; 95 percent CI, 1.11–2.78; p=0.02). [N Engl J Med 2016;375:1834-1844] “The increased risk of retinopathy could be attributed to a rapid and marked decline in HbA1c [during the first 16 weeks of treatment in patients with preexisting diabetic retinopathy and poor glycaemic control at baseline, especially those on insulin treatment],” Lovshin explained. [Diabetes Obes Metab 2018;20:889-897]
Semaglutide benefits in real world
“In the real-world setting, [switching from another GLP-1 RA to] SC semaglutide Q1W demonstrated statistically significant improvements in HbA1c and body weight,” noted Lovshin. [Rudofsky G, et al, EASD 2023, abstract 76-LB]
A post hoc analysis of the real-world, multinational SURE programme evaluated the efficacy and safety of switching to SC semaglutide Q1W from another GLP-1 RA, including liraglutide and dulaglutide, among 651 T2DM patients (mean age, 60 years; male, 53 percent; mean duration of T2DM, 13.1 years). At week 30, mean HbA1c was reduced by 0.6 percent-points (n=620; 7.2 percent vs 7.8 percent at baseline; 95 percent CI, -0.68 to -0.54; p<0.0001), and mean body weight was reduced by 3.0 kg (n=633; 99.0 kg vs 102.0 kg; 95 percent CI, -3.33 to -2.64; p<0.0001).
In the SURE programme (n=3,505), no new safety concerns with SC semaglutide Q1W were noted. The most commonly reported adverse events (AEs) were gastrointestinal (GI) disorders (16.3 percent), with only 0.4 percent being serious AEs. [Yale JF, et al, EASD 2023, abstract 797-P]
Addressing GLP-1 RAs’ safety concerns
“In my clinical experience, 10–20 percent of patients on semaglutide encounter GI AEs, most of which is nausea,” noted Lovshin. “Slow titration [of semaglutide] may help reduce GI AEs and improve tolerance.”
Addressing a nest case-control study on the risk of thyroid cancer associated with GLP-1 RA use, Lovshin pointed out that the mechanism of GLP-1 RA on medullary thyroid carcinoma was established based on rodent models. Thyroid C-cells in humans exhibit lower levels of GLP- 1 receptors than those in rodents. [Diabetes Care 2023;46:384-390; J Clin Endocrinol Metab 2011;96:2027- 2031] “In our clinical practice, we have encountered no thyroid [C-cell] tumours,” Lovshin said.
Similarly, while animal studies suggested that GLP-1 RAs may increase proliferation of pancreatic cells, clinical studies showed no association between GLP-1 RA use and pancreatic cancer or acute pancreatitis. [Curr Diab Rep 2016;16:120; Cleve Clin J Med 2022;89:457-464]
“Nevertheless, GLP-1 RAs should be avoided in patients with a history of pancreatitis [or pancreatic cancer]. It is important to assess patients’ medical history, inform them of potential risks, and engage in shared decision-making,” Lovshin advised.
International guidelines on GLP-1 RAs
“GLP-1 RAs are recommended for T2DM patients with established CVD or CV risk factors,” said Lovshin.
The 2020 update of Diabetes Canada’s Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada recommends that a GLP-1 RA with proven CV benefit should be considered in T2DM patients with atherosclerotic CVD (ASCVD) and those aged ≥60 years with ≥2 CV risk factors. [Can J Diabetes 2020;44:575-591]
2022 joint consensus guidelines of the American Diabetes Association (ADA) and EASD recommend the use of a GLP-1 RA with proven CV benefit in T2DM patients with established ASCVD or high-risk factors. The decision to use a GLP-1 RA should be independent of baseline HbA1c. [Diabetes Care 2022;45:2753-2786]
The ADA’s 2023 Guidelines for Diabetes Care recommend use of a GLP-1 RA with proven CV benefit in T2DM patients with ASCVD or high CV risk, independent of HbA1c and metformin use. In weight management, a glucose-lowering therapy with high to very high weight-loss efficacy, such as semaglutide, should be considered. [Diabetes Care 2023;46:S140-S157]
The European Society of Cardiology̓s 2023 CVD guidelines recommend treatment with GLP-1 RAs for T2DM patients with ASCVD, independent of HbA1c and other concomitant glucose-lowering medication. [Eur Heart J 2023;44:4043-4140]
“In patients with established ASCVD, including [ischaemic] stroke, a GLP-1 RA should be added to treatment [with metformin, regardless of baseline HbA1c],” Lovshin added. [Stroke 2021;52:e364-e467]