Metabolic dysfunction–associated steatotic liver disease (MASLD) can lead to the development of metabolic dysfunction–associated steatohepatitis (MASH), cirrhosis, and hepatocellular carcinoma (HCC). Hong Kong studies show that sodium-glucose cotransporter 2 inhibitors (SGLT2i), such as empagliflozin, can reduce liver fat in nondiabetic patients with MASLD and lower the risk of HCC in patients with coexisting type 2 diabetes (T2D) and chronic hepatitis B (CHB).
MASLD epidemic – a rising risk factor for HCC
MASLD has become an epidemic globally, with an estimated prevalence of 32.4 percent. [Lancet Gastroenterol Hepatol 2022;7:851-861] In Asia, MASLD affects 29.6 percent of the population, with an incidence rate of 50.9 cases per 1,000 person-years. [Lancet Gastroenterol Hepatol 2019;4:389-398]
According to a recent report by the Lancet Commission on addressing the global HCC burden, the incidence of MASLD-related HCC is expected to grow in the next decade, particularly in Asia, Europe and the US, due to increasing rates of obesity. [Lancet 2025;406:731-778]
“While hepatitis B virus [HBV] will remain the leading cause of liver cancer in 2050, its proportion is expected to decrease from 39 percent in 2022 to 36.9 percent in 2050. Similarly, the proportion of liver cancer cases caused by hepatitis C virus [HCV] will decline from 29.1 percent in 2022 to 25.9 percent in 2050,” wrote the authors of the Commission’s report.
In contrast, MASH as a cause of HCC is projected to rise, from accounting for 8 percent of the cases in 2022 to 11 percent of the cases in 2050.
“We estimate that ≥60 percent of liver cancer cases are preventable via control of modifiable risk factors, including HBV, HCV, MASLD, and alcohol,” the report’s authors highlighted.
Empagliflozin reduces liver fat in nondiabetic MASLD patients
A double-blind, randomized, placebo-controlled trial initiated by researchers from the University of Hong Kong (HKU) showed that empagliflozin treatment significantly reduced hepatic fat content vs placebo in nondiabetic patients with MASLD. [Hepatology 2024;80:916-927] A recently published analysis of stool samples from patients in the empagliflozin group suggests that gut microbiota may predict treatment response. [Liver Int 2025;45:e70023]
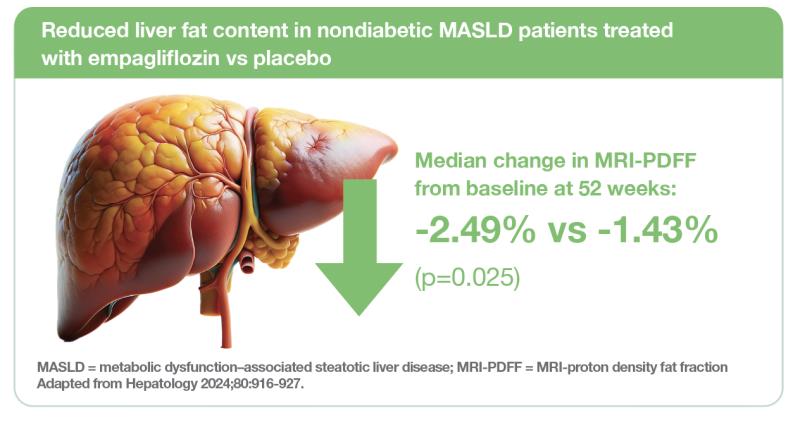
The prospective cohort study included 98 nondiabetic patients with MALSD who had MRI-proton density fat fraction (MRI-PDFF; the most accurate noninvasive test for quantifying liver fat content) of ≥5 percent (median age, 55.7 years; male, 55.1 percent; median BMI, 27.4 kg/m2; overweight or obese, 93.9 percent). The participants, recruited from the community, were randomized 1:1 to receive empagliflozin 10 mg daily (n=49; median MRI-PDFF, 9.7 percent) or placebo (n=48; median MRI-PDFF, 9.0 percent) for 52 weeks. [Hepatology 2024;80:916-927]
End-of-treatment assessment at 52 weeks showed significantly greater reduction in median MRI-PDFF from baseline in the empagliflozin vs placebo group (-2.49 vs -1.43 percent; p=0.025), thus meeting the trial’s primary endpoint.
Subgroup analysis showed a significantly greater decrease in median MRI-PDFF in the empagliflozin arm among:
- Participants aged ≥60 years (-2.40 vs -0.89 percent for empagliflozin vs placebo; p=0.025);
- Female participants (-2.76 vs -0.97 percent; p=0.012);
- Participants with overweight or obesity (-2.47 vs -1.10 percent; p=0.019); and
- Participants with baseline alanine aminotransaminase (ALT) ≤30 U/L (-2.46 vs -1.09 percent; p=0.005).
Resolution of hepatic steatosis (ie, MRI-PDFF < 5 percent), a secondary outcome, showed a nonsignificant trend favouring empagliflozin vs placebo (44.9 vs 28.6 percent; p=0.094) at week 52.
No significant between-group differences were observed in other liver-related secondary outcomes, including ALT drop ≥17 U/L (16.3 vs 12.2 percent; p=0.564), MRI-PDFF drop ≥30 percent (49.0 vs 40.8 percent; p=0.417), and a composite outcome of both (8.2 vs 8.2 percent; p=1.000).
At 52 weeks, patients in the empagliflozin group had significantly greater median reduction of body weight (-2.7 vs -0.2 kg [-3.6 vs -0.3 percent]; p<0.001) vs those in the placebo group. Decreases in waist circumference (-2.0 vs 0 cm), fasting glucose (-0.3 vs 0 mmol/L) and ferritin (-126 vs -22 pmol/L) were also significantly greater with empagliflozin vs placebo at week 52 (all p<0.05).
“The significantly greater reduction in ferritin levels in the empagliflozin group supports its potentially beneficial effects in alleviating liver inflammation in MASLD,” the researchers suggested.
Gut microbiota may predict MASLD response to empagliflozin
In the above trial, stool samples were collected at baseline and end of treatment (52 weeks) in the empagliflozin group. These stool samples were analyzed with shotgun DNA metagenomic sequencing to investigate the association between gut microbiota composition and response to empagliflozin treatment in nondiabetic MASLD patients. [Liver Int 2025;45:e70023]
Results showed significant difference in alpha diversity (Shannon index, p<0.001; Simpson index, p=0.001) and beta diversity (p=0.048) in baseline microbiome between treatment responders (n=22) and nonresponders (n=23), indicating that gut dysbiosis may hinder MASLD response to empagliflozin treatment.
Six gut microbial species were enriched in treatment responders:
- Faecalibacterium prausnitzii (log10LDA score, 4.27);
- Lachnospira pectinoschiza (log10LDA score, 3.99);
- Anaerostipes hadrus (log10LDA score, 3.98);
- Roseburia faecis (log10LDA score, 3.97);
- Roseburia inulinivorans (log10LDA score, 3.58); and
- Agathobaculum butyriciproducens (log10LDA score, 2.77).
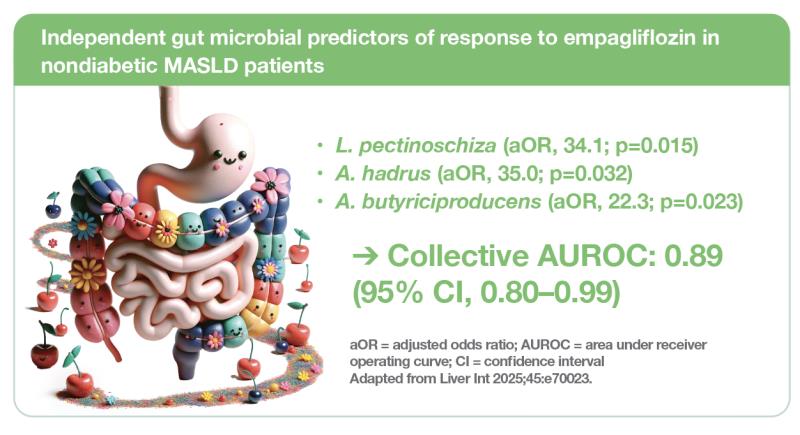
Three of the bacterial species, namely, L. pectinoschiza (adjusted odds ratio [aOR], 34.1; p=0.015), A. hadrus (aOR, 35.0; p=0.032) and A. butyriciproducens (aOR, 22.3; p=0.023), independently predicted treatment response, while clinical factors did not.
“These three species collectively predicted treatment response with an area under receiver operating curve [AUROC] of 0.89 [95 percent confidence interval (CI), 0.80–0.99],” the researchers reported.
However, no significant change was found in the relative abundance of the six putative gut bacterial species between baseline and 1 year in both treatment responders and nonresponders.
Empagliflozin well tolerated in nondiabetic MASLD
Empagliflozin was well tolerated in the above HKU trial involving nondiabetic MASLD patients. Adverse events (AEs) occurred at low frequencies and were all transient even without treatment cessation. No significant differences in rates of AEs were observed between the empagliflozin and placebo groups. [Hepatology 2024;80:916-927]
Potential role in nondiabetic MASLD?
“Using empagliflozin for 52 weeks significantly reduced liver fat content in nondiabetic MASLD patients, and was associated with reductions in serum ferritin, fasting glucose and anthropometric parameters as well as a good safety profile,” the HKU researchers concluded. “Further large-scale, multicentre, randomized controlled trials in diverse populations are needed to verify our results, which could potentially extend empagliflozin’s role to treating nondiabetic MASLD, particularly in patients with MASH and advanced fibrosis or cirrhosis.” [Hepatology 2024;80:916-927]
“Certain gut bacterial species, particularly the combination of L. pectinoschiza, A. hadrus and A. butyriciproducens, may predict response to empagliflozin treatment in nondiabetic MASLD patients,” they noted. [Liver Int 2025;45:e70023]
SGLT2i reduce HCC risk in patients with T2D and CHB
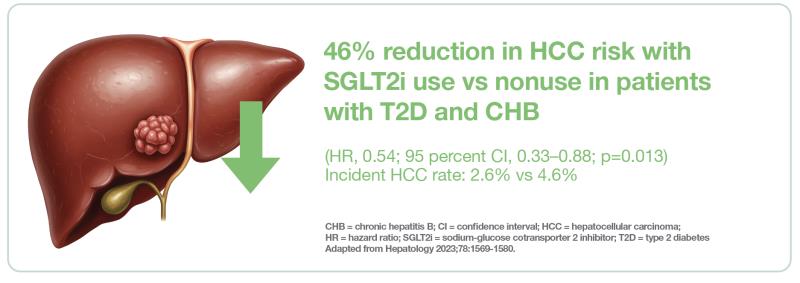
A territory-wide cohort study by researchers from HKU showed that SGLT2i use is associated with a reduced risk of HCC among patients with coexisting T2D and CHB. [Hepatology 2023;78:1569-1580]
Notably, the protective effect against HCC was consistently observed among SGLT2i users vs nonusers regardless of sex, age, level of glycaemic control, T2D duration, presence of hepatic steatosis or cirrhosis, timing of nucleos(t)ide analogue (NA) therapy with respect to baseline, and background use of dipeptidyl peptidase-4 inhibitors (DPP4i’s), insulin, or glitazones.
In this real-world population-based study using data from the Hospital Authority’s electronic medical record database, researchers identified 14,638 patients aged 20–85 years who had coexisting T2D and CHB in 2015–2020. After propensity score matching, 2,000 patients (1,000 SGLT2i users and 1,000 nonusers) were included for analysis.
The included patients (mean age, 60.9 years; male, 71.3 percent; mean BMI, 26.2 kg/m2) had longstanding T2D (mean duration, 12.6 years) and suboptimal glycaemic control (mean HbA1c, 8.5 percent) at baseline. A majority were on metformin (87.3 percent) and sulphonylureas (64.5 percent).
Among patients on NAs at baseline, mean treatment duration was 59.5 months, and entecavir was the most commonly used agent (82.8 percent). Hepatic steatosis was present in more than half of the patients (56.5 vs 52.4 percent of SGLT2i users vs nonusers), while cirrhosis was present in about a quarter of the patients (23.5 vs 25.2 percent).
Empagliflozin was the most commonly used SGLT2i (65.1 percent) in the study cohort.
After a median follow-up of 17 months, SGLT2i users were found to have a significant 46 percent reduction in risk of HCC vs nonusers (hazard ratio [HR], 0.54; 95 percent CI, 0.33–0.88; p=0.013). A total of 26 vs 46 cases of incident HCC (2.6 vs 4.6 percent) were reported in the SGLT2i user vs nonuser group, corresponding to incidence rates of 1.39 vs 2.52 cases per 100 person-years.
Subgroup analysis showed consistent results (all pinteraction>0.05) regardless of:
- Sex (HR, 0.48 for men vs 0.94 for women);
- Age (HR, 0.70 for <65 years vs 0.38 for ≥65 years);
- HbA1c (HR, 0.44 for <7.5 percent vs 0.52 for ≥75 percent);
- Duration of diabetes (HR, 0.24 for <10 years vs 0.71 for ≥10 years);
- Cirrhosis status at baseline (HR, 0.48 for with cirrhosis vs 0.55 for without cirrhosis);
- Hepatic steatosis status at baseline (HR, 0.58 for with hepatic steatosis vs 0.92 for without hepatic steatosis);
- Timing of anti-HBV therapy (HR, 0.49 for initiation at or before baseline vs 0.65 for initiation during the observation period);
- DPP4i use at baseline (HR, 0.48 for DPP4i use vs 0.60 for nonuse);
- Insulin use at baseline (HR, 0.84 for use vs 0.35 for nonuse); and
- Glitazone use at baseline (HR, 0.64 for nonuse; HR not applicable to those with glitazone use due to only 1 HCC event in the SGLT2i user group).
“[These findings are] important because in real-world clinical practice, T2D patients often require different combinations of antidiabetic agents for glycaemic control based on their comorbidities,” the researchers commented.
“Of note, the inverse correlation between SGLT2i use and HCC was independent of cirrhosis, which is present in a high proportion of patients with coexisting T2D and CHB,” they added.
As CHB patients with more advanced age, diabetes and cirrhosis have a high predicted risk of HCC, the researchers suggested that their findings should prompt prospective clinical studies to confirm the antitumour effect of SGLT2i in this special group of patients.
Potential HCC-protective role in patients with T2D and CHB?
“To our knowledge, this is the first real-world population-wide cohort study examining the association between SGLT2i use and risk of developing HCC among exclusively patients with coexisting T2D and CHB, with the majority of patients already put on anti-HBV therapy with entecavir at baseline,” the researchers noted. [Hepatology 2023;78:1569-1580]
“The 46 percent reduction in HCC risk shown in SGLT2i users vs nonusers, together with the consistent protective effect observed across different subgroups, indicate a potential hepatic benefit of SGLT2i in addition to their cardiorenal protective effects,” they suggested, while adding that further studies are required for validation and confirmation of these findings.