Management of DKD: Current knowledge and practical insights









Diabetic kidney disease (DKD) is a global healthcare burden. At a symposium co-organized by Diabetes Hongkong and Hong Kong Society of Nephrology, Professor Ronald Ma of the Chinese University of Hong Kong discussed the current landscape of DKD in Hong Kong, and Dr Masayuki Yamanouchi, a specialist in nephrology from Toranomon Hospital in Tokyo, Japan, discussed clinical and real-world evidence on the effects of a nonsteroidal mineralocorticoid receptor antagonist (MRA), finerenone, in improving outcomes in patients with type 2 diabetes mellitus (T2DM) and chronic kidney disease (CKD). Dr Yamanouchi also presented two patient cases to illustrate the clinical benefits of finerenone in DKD patients with different disease severity.
High burden of DKD
Diabetes affects 537 million people worldwide, with 90 percent attributed to T2DM, which is associated with an increased risk of cardiovascular (CV) events and CKD, potentially leading to end-stage renal disease (ESRD). [International Diabetes Federation (IDF) Diabetes Atlas 10th edition; Kidney Int 2003;63:225-232]
“Unfortunately, Hong Kong ranks the highest in the world for the percentage of incident cases of treated ESRD [52.4 percent] that are attributed to diabetes, meaning the burden of DKD is very high in Hong Kong,” said Ma. [US Renal Data System (USRDS) 2022 Annual Data Report]
Young-onset diabetes a major concern
Data from the Hong Kong Diabetes Surveillance Database indicate that ESRD incidence increases with increasing duration of diabetes. In patients with diabetes for ≤2 years, incidence of ESRD is 4/1,000 person-years (PY), while a >5-fold increase in ESRD incidence is observed in patients with diabetes for ≥15 years, reaching 22/1,000 PY. More importantly, approximately 2 percent of patients with diabetes for ≥10 years develop ESRD every year. [Diabetes Care 2017;40:928-935]
Young-onset diabetes (YOD) diagnosed at <40 years of age is associated with higher risks for cardiovascular and renal complications that are partly driven by longer disease duration. “YOD is becoming a major concern because of its alarmingly high prevalence worldwide and in Asia. According to the Hong Kong Diabetes Register, 21 percent of patients with diabetes from the Hong Kong Diabetes Register have YOD – a rate similar to other regions in Asia,” Ma commented. [Am J Med 2014;127:616-624; Lancet Diabetes Endocrinol 2014:935-943]
Heterogeneity of renal function decline in DKD
DKD manifests as decline in estimated glomerular filtration rate (eGFR) and/or albuminuria, and both components are critical for DKD classification and prognosis determination. [Kidney Int Rep 2023;9:323-333]
Data from the Hong Kong Diabetes Register reveal that while some DKD patients have preserved or slowly declining renal function, a significant proportion of patients have rapid eGFR decline of >4 percent per year. [Kidney Int 2016;89:411-420] A latent trajectory analysis has identified four subtypes of eGFR trajectories: gradual (84 percent of patients), curvilinear (7 percent), progressive (6 percent), and accelerated (3 percent) eGFR decline. [Kidney Int 2019;95:178-187]
“These findings highlight the need to recognize the heterogeneity within a phenotype and identify individuals at high risk of DKD, which is in line with the concept of precision medicine,” Ma noted.
Albuminuria or reduced eGFR alone spells higher risks of CV disease and CKD progression
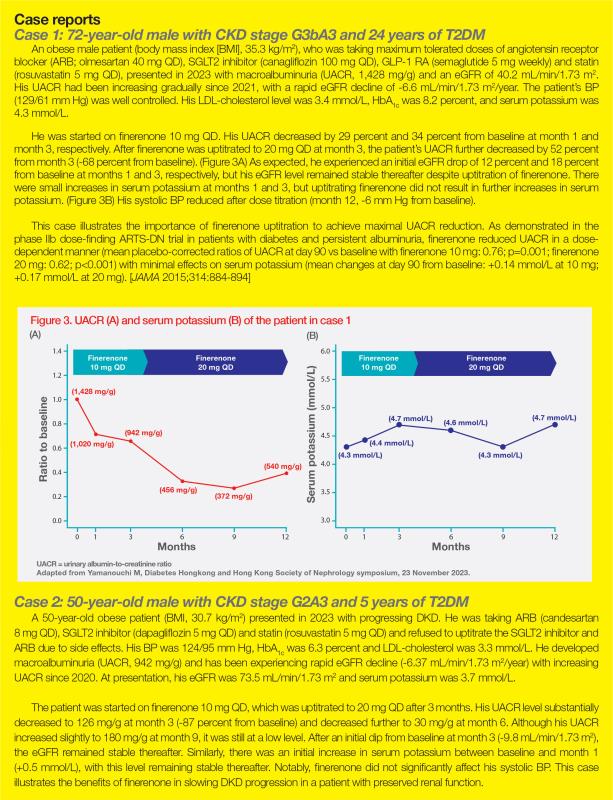
A cohort study from the Hong Kong Diabetes Biobank included 19,025 T2DM patients with four clinical phenotypes at baseline: no evidence of DKD (51 percent of patients), albuminuria only (29 percent), reduced eGFR (<60 mL/min/1.73 m2) only (5 percent), and albuminuria plus reduced eGFR (15 percent). Predictably, patients with albuminuria plus reduced eGFR had the worst cardiorenal outcomes. Notably, patients with normoalbuminuric DKD with reduced eGFR or albuminuric DKD with preserved renal function had higher risks of CV disease and CKD progression than those with no DKD, regardless of baseline eGFR, meaning that either albuminuria or reduced eGFR alone is predictive of higher risk of CV disease and CKD progression. (Figure 1) [Am J Kidney Dis 2022;80:196-206.e1]
A longitudinal cohort study (1990–2020) in Japan found that 49 percent of 319 biopsy-confirmed DKD patients progressed to ESRD after a median follow-up of 3.3 years, and 92 percent of all DKD patients exhibited a curvilinear pattern in their eGFR trajectory. “We also found high variability in urinary albumin-to-creatinine ratio [UACR] [median coefficient of variation for UACR, 48.9] with a median compound annual growth rate of 43.6 percent for UACR and a positive association between the instantaneous speed of eGFR decline and UACR. These findings highlight the importance of close monitoring of UACR fluctuation over time in patients with DKD,” said Yamanouchi. [Kidney Int Rep 2023;9:323-333]
“Therefore, other than renal function tests, regular testing for albuminuria, which is often neglected in practice, is essential in patients with diabetes, as it provides a unique window of opportunity for early diagnosis and intervention as well as optimization of clinical outcomes,” emphasized Ma.
Other biomarkers of DKD progression
A consecutive cohort of 9,096 patients with T2DM from the Hong Kong Diabetes Register showed that patients with acute kidney injury (AKI) were 14 times and 12 times more likely to develop incident CKD (hazard ratio [HR], 14.3; 95 percent confidence interval [CI], 12.69–16.11) and ESRD (HR, 12.1; 95 percent CI, 10.74–13.62), respectively, vs patients without AKI after 12 years of follow-up. Patients with more severe AKI and poorer recovery from AKI were also more likely to progress to ESRD over time. In addition, serum uric acid was independently associated with incident CKD (HR, 4.13; 95 percent CI, 2.9–5.89), ESRD (HR, 12.96; 95 percent CI, 8.11–20.7) and all-cause mortality (HR, 2.48; 95 percent CI, 1.6–3.82). [Diabetes 2022;71:520-529]
Emerging biomarkers may also help facilitate risk stratification in DKD. Research links DNA methylation markers with renal function and deterioration in kidney function in T2DM patients. [Nat Commun 2023;14:2543] In another study, serum haemoglobin concentration negatively correlates with renal pathology changes in diabetes patients, which may be a potential biomarker for predicting DKD progression in early stages of the disease. [Nephrol Dial Transplant 2022;37:489-497]
Underlying mechanisms of DKD and CVD progression
DKD and CVD progression in patients with CKD and T2DM is driven by a combination of haemodynamic, metabolic, inflammatory and fibrotic factors. [Clin J Am Soc Nephrol 2017;12:2032-2045]
Current therapies for patients with DKD primarily target the haemodynamic and metabolic factors. Sodium-glucose cotransporter 2 (SGLT2) inhibitors decrease glomerular hypertension by vasodilation of the post-glomerular arteriole. Renin-angiotensin-system (RAS) inhibitors impact the renal vasculature and renal haemodynamic function in patients with DKD. In addition, SGLT2 inhibitors and glucagon-like peptide-1 receptor agonists (GLP-1 RAs) have favourable metabolic effects, including weight loss and glycaemic control, in patients with T2DM. [Nephrology (Carlton) 2021;26:377-390; Sci Rep 2020;10:12837]
Phase III randomized controlled trials (RCTs) have demonstrated that an SGLT2 inhibitor plus a RAS inhibitor can offer kidney protection and lower the risk of CV events. However, after a median follow-up of 2.5 years, CKD progression and CV events still occurred in 5–8 percent of patients treated with this combination. [N Engl J Med 2019;380:2295-2306; N Engl J Med 2020;383:1436-1446]
“The substantial residual cardiorenal risk in DKD is thought to be attributed to chronic inflammation, which is not the primary target of SGLT2 inhibitors and RAS inhibitors, thus highlighting a need for further treatment options,” commented Yamanouchi. [Adv Chronic Kidney Dis 2018;25:181-191]
Targeting inflammation and fibrosis in DKD
Overactivation of mineralocorticoid receptor (MR) has been implicated as a driver of inflammation and fibrosis in DKD. Finerenone is a nonsteroidal MRA that is thought to inhibit inflammation and fibrosis mediated by MR overactivation, which leads to kidney and CV damage. [Front Pharmacol 2021;12:754239; Nephrol Dial Transplant 2022;37:1014-1023]
“Finerenone has a higher potency against MR than steroidal MRAs, such as eplerenone, and a higher selectivity for MR than both spironolactone and eplerenone, which results in fewer sexual side effects, such as gynaecomastia. It has a shorter half-life of 2–3 hours and exerts a modest effect in reducing blood pressure [BP] compared with steroidal MRAs,” said Yamanouchi. [Kerendia Hong Kong Prescribing Information, July 2022; Spironolactone SmPC, February 2022; Eplerenone SmPC, November 2022; Am J Hypertens 2023;36:135-143]
Clinical evidence of nonsteroidal MRA in DKD
FIDELIO-DKD (n=5,734) and FIGARO-DKD (n=7,437) were two phase III RCTs that investigated the efficacy and safety of finerenone in addition to the maximum tolerated dose of a RAS inhibitor in T2DM patients with mild-to-severe CKD. The two studies had a similar design but different primary efficacy endpoints, with a kidney composite endpoint (kidney failure, a sustained decline in eGFR of ≥40 percent from baseline or renal death) in FIDELIO-DKD and a CV composite endpoint (CV death, nonfatal myocardial infarction, nonfatal stroke or hospitalization for HF [HHF]) in FIGARO-DKD. [N Engl J Med 2020;383:2219-2229; N Engl J Med 2021;385:2252-2263]
After a median follow-up of 3 years, a prespecified pooled analysis of the two trials (FIDELITY) showed that finerenone significantly reduced the risk of the kidney composite outcome (time to kidney failure, sustained eGFR decline of ≥57 percent or renal death) by 23 percent vs placebo (HR, 0.77; 95 percent CI, 0.67–0.88; p=0.0002). Significant reductions in sustained eGFR decline (HR, 0.70; 95 percent CI, 0.60–0.83; p<0.0001) and ESRD (HR, 0.80; 95 percent CI, 0.64–0.99; p=0.04) were observed. The kidney benefits were consistent regardless of baseline eGFR or UACR. [Eur Heart J 2022;43:474-484]
“Finerenone reduced UACR rapidly, with a 32 percent lower least squares [LS] mean change from baseline vs placebo at month 4 [ratio of LS mean, 0.68; p<0.0001], and this effect was maintained throughout the study,” Yamanouchi noted. [Eur Heart J 2022;43:474-484]
In the overall population, the LS mean change in eGFR from baseline to end of treatment was similar between the finerenone and placebo groups (-3.5 mL/min/1.73 m2 vs -3.6 mL/min/1.73 m2). Despite an initial acute eGFR decline observed shortly after finerenone initiation, finerenone-treated patients exhibited slower chronic eGFR decline vs placebo recipients in the long term (from month 4 to end of treatment, -2.5 mL/min/1.73 m2/year vs -3.7 mL/min/1.73 m2/year; p<0.0001), regardless of baseline eGFR. [Kidney Int 2023;103:196-206]
Finerenone also significantly reduced the risk of the CV composite outcome by 14 percent vs placebo (HR, 0.86; 95 percent CI, 0.78–0.95; p=0.0018). A significantly lower incidence of HHF was reported with finerenone vs placebo (HR, 0.78; 95 percent CI, 0.66–0.92; p=0.0030). Finerenone’s cardiorenal benefits were consistent regardless of baseline eGFR or UACR or SGLT2 inhibitor or GLP-1 RA use at baseline. [Eur Heart J 2022;43:474-484; Diabetes Care 2022;45:2991-2998; Diabetes Obes Metab 2023;25:407-416]
Finerenone was associated with a minimal increase in mean serum potassium concentration (+0.19 mmol/L between finerenone and placebo at month 4), and serum potassium level became stable thereafter. Although investigator-reported hyperkalaemia occurred more frequently with finerenone vs placebo (14.0 percent vs 6.9 percent), the clinical impact of hyperkalaemia was minimal, with no hyperkalaemia-related deaths and only 1.7 percent of patients in the finerenone group discontinuing their participation in the study due to hyperkalaemia. [Eur Heart J 2022;43:474-484]
Clinical guidelines on DKD management
Recent clinical trials in patients with T2DM and CKD suggest that combining SGLT2 inhibitor and finerenone plus RAS inhibitor may delay kidney function decline. For patients with T2DM and CKD, the American Diabetes Association and Kidney Disease: Improving Global Outcomes (ADA/KDIGO) consensus recommends use of an SGLT2 inhibitor in patients with ≥20 mL/min/1.73 m2 to reduce CKD progression. It also recommends a nonsteroidal MRA for patients with T2DM and an eGFR ≥25 mL/min/1.73 m2, normal serum potassium level and albuminuria (UACR ≥30 mg/g) despite maximum tolerated dose of RAS inhibitor. [Nat Rev Nephrol 2022;18:78-79; Diabetes Care 2022;45:3075-3090] Due to limited data from RCTs, the European Renal Association recommends that physicians should use clinical judgement and a personalized approach when deciding if an SGLT2 inhibitor or finerenone should be started first whilst allowing 4–6 weeks between initiating either agent for repeat checking of BP, eGFR and serum potassium. [Clin Kidney J 2023;16:1885-1907]
Real-world evidence on nonsteroidal MRA in DKD
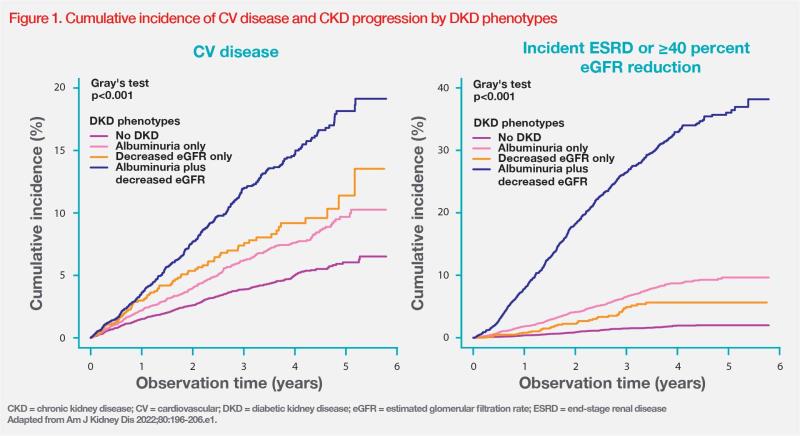
Yamanouchi’s ongoing real-world Japanese cohort study has recruited >100 patients with T2DM and CKD since 2022. The study is investigating the effects of finerenone on UACR, eGFR and serum potassium. Results of the cohort analysis (n=83) showed that 83.1 percent of patients were on a RAS inhibitor and 89.2 percent were on an SGLT2 inhibitor at baseline. Mean BP was 128/73 mm Hg, mean HbA1c was 7.0 percent and mean serum potassium was 4.3 mmol/L. [Yamanouchi M, et al, manuscript in preparation]
“Forty-eight percent of patients had macroalbuminuria at baseline. The median UACR was 329 mg/g and median eGFR was 38.1 mL/min/1.73 m2 for all patients,” reported Yamanouchi. “Results also showed effective reduction of UACR by 25–40 percent with LS mean reduction in eGFR of approximately 4 mL/min/1.73 m2 from baseline to month 12 with finerenone, which is similar to the magnitude of eGFR reduction at month 12 observed in FIDELITY. This analysis also showed minimal changes in serum potassium and systolic BP.” (Figure 2) [Yamanouchi M, et al, manuscript in preparation]
“To manage the initial eGFR dip associated with finerenone, we can take reference from the current KDIGO guideline, which suggests reviewing the causes of AKI, correcting volume depletion, reassessing concomitant medications, such as diuretics and nonsteroidal anti-inflammatory drugs, and considering renal artery stenosis in case of >30 percent increase in creatinine,” advised Yamanouchi.