
Despite the success of COVID-19 vaccines, immunocompromised individuals, older adults and those with comorbidities remain at disproportionately high risk of severe outcomes from COVID-19. This underscores the need for additional preventive measures for these vulnerable populations. At the Advances in Medicine (AIM) 2024 conference, Professor Barnaby Young from the National Centre for Infectious Diseases, Singapore, shared insights on current COVID-19 management practices, including the important role of antivirals, such as remdesivir (Veklury®, Gilead), in treatment of vulnerable patients.
Double burden of COVID-19 and comorbidities
“With vaccines and the emergence of Omicron, COVID-19 has evolved into a less severe and is often an incidental finding of uncertain clinical significance in patients admitted to hospital during an outbreak,” noted Young.
Despite this, COVID-19 remains an infection that can cause serious illness. A cohort study using data from the US Department of Veterans Affairs evaluated the risk of death in patients hospitalized for COVID-19 (n=8,625) or seasonal influenza (n=2,647) following the emergence of the JN.1 variant in winter 2023. The death rate at 30 days was 5.70 percent for COVID-19 vs 4.24 percent for influenza (adjusted hazard ratio, [HR], 1.35; 95 percent confidence interval [CI], 1.10–1.16). [JAMA 2024:e247395]
“The risk of death from COVID-19 increases with age and the number of comorbidities that a patient has, even among those who are vaccinated,” pointed out Young. The population-based INFORM study revealed that, despite receiving ≥4 vaccine doses, immunocompromised individuals (including those with end-stage kidney disease [ESKD] on dialysis) remained at high risk of COVID-19 hospitalization and death. [Dube S, et al, ECCMID 2024, abstract P0409] Furthermore, a post hoc analysis of real-world COVIDRIVE data (n=5,280) showed that chronic kidney disease (CKD; 17.9 vs 16.7 percent) and immunocompromising conditions (14.2 vs 13.6 percent) were more prevalent among hospitalized patients with a severe acute respiratory illness who tested positive vs negative for SARS-CoV-2. [Meeraus W, et al, ECCMID 2024, abstract P0382]
Management of COVID-19
Management of COVID-19 involves three steps. First, active protection is acquired through vaccination. Next, for people with inadequate immune response to vaccines, such as the immunocompromised, passive immunity can be achieved with monoclonal antibodies (mAbs). Third, antivirals are administered early in the disease course to ensure optimal clinical benefit.
“While COVID-19 has changed dramatically since 2020, the cornerstones of the therapeutic management of hospitalized adults with COVID-19 were established in pivotal trials in mid-2020,” pointed out Young. “For immunocompromised patients who are at high risk of progressing to severe COVID-19, remdesivir is the preferred antiviral therapy. The addition of dexamethasone and other immunomodulators is recommended as disease severity increases.” [NIH, 2024, Coronavirus Disease 2019 (COVID-19) Treatment Guidelines, https://www.covid19treatmentguidelines.nih.gov/]
Of note, with the emergence and continued evolution of Omicron, most mAbs have lost their ability to neutralize currently circulating SARS-CoV-2 variants. However, the available antiviral drugs, namely, remdesivir, nirmatrelvir-ritonavir, and molnupiravir, continue to be effective. [Nat Commun 2024;15:2254] A retrospective cohort study (n=143,338) among highly vaccinated adult Singaporeans aged ≥60 years with SARS-CoV-2 infection in the community showed that treatment with nirmatrelvir-ritonavir significantly reduced the risk of hospitalization. [Clin Microbiol Infect 2023;29:1328-1333]
Safety concerns with nirmatrelvir-ritonavir use
An important drawback of nirmatrelvir-ritonavir is the potential for significant drug-drug interactions (DDIs) with comedications. Ritonavir boosts plasma concentrations of nirmatrelvir via potent and rapid inhibition of the cytochrome P450 (CYP) 3A4 isoenzyme, which is responsible for nirmatrelvir metabolism. This can lead to substantially increased plasma concentrations of concurrent drugs predominantly metabolized through this pathway. [Clin Pharmacol Ther 2022;112:1191-1200]
“Different DDIs have to be considered before prescribing nirmatrelvir-ritonavir,” said Young (Figure) “With the advice and support of inpatient pharmacists, it is possible to monitor or withhold administration [or adjust dosages] of comedications when prescribing nirmatrelvir-ritonavir in the hospital setting, but this is not so easy to implement in the community setting.”
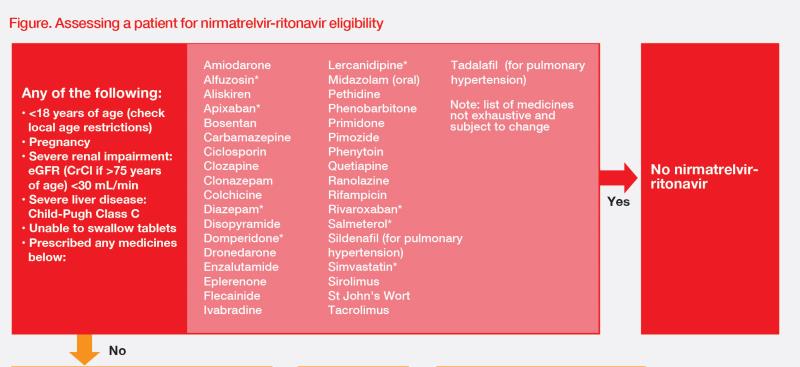
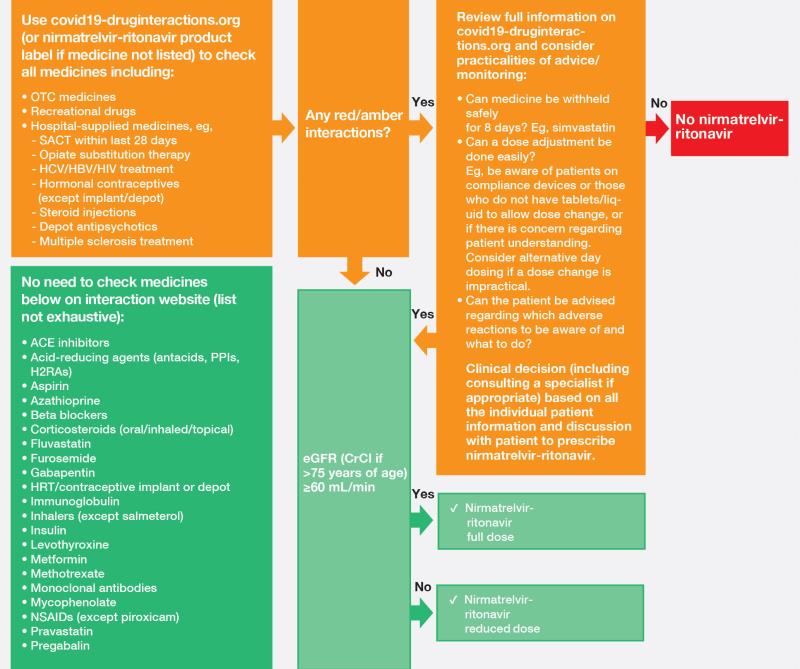

“In addition, nirmatrelvir-ritonavir is not recommended for patients with severe renal insufficiency [estimated glomerular filtration rate (eGFR) <30 mL/min/1.73m2 or on dialysis],” he added. Dose adjustments are required for patients with moderate renal impairment (eGFR ≥30–<60 mL/min/1.73 m2). [NIH, 2024, Coronavirus Disease 2019 (COVID-19) Treatment Guidelines, https://www.covid19treatmentguidelines.nih.gov/]
Remdesivir suitable for patients with any eGFR
In contrast to nirmatrelvir-ritonavir, remdesivir may be used without dose adjustment in patients with severe renal impairment, including those receiving dialysis. [NIH, 2024, Coronavirus Disease 2019 (COVID-19) Treatment Guidelines, https://www.covid19treatmentguidelines.nih.gov/]
REDPINE study
REDPINE was a randomized, double-blind, phase III trial of remdesivir vs placebo in adults (n=243) with moderately or severely reduced kidney function who were hospitalized with severe COVID-19 pneumonia. Overall, 37 percent of patients had acute kidney injury, 26 percent had CKD, and 37 percent had ESKD requiring chronic dialysis. [Santos JR, et al, ECCMID 2023, abstract P2635]
“There was no increased risk of adverse events amongst the group that received remdesivir vs placebo, regardless of the type of renal impairment,” pointed out Young. Adverse events related to study drug were reported in 8 and 4 percent of patients treated with remdesivir and placebo, respectively. The trial was underpowered for efficacy as it was terminated early due to challenges with recruitment.
Summary
COVID-19 continues to evolve, likely towards becoming a seasonal infection. However, immunocompromised patients, including those with renal impairment, are at increased risk of severe COVID-19, which requires additional measures of prevention. It requires intravenous administration, but remdesivir is not associated with significant DDIs and can be administered to high-risk patients regardless of kidney disease status. Phase III trial data have demonstrated remdesivir’s tolerability in patients with moderately or severely reduced kidney function.