Maximizing outcomes in nAMD and PCV with a multitargeted VEGF inhibitor









The anti–vascular endothelial growth factor (anti-VEGF), aflibercept, has demonstrated durable visual improvements in patients with various exudative retinal diseases. At a Bayer-sponsored symposium during the 16th Congress of the Asia-Pacific Vitreo-Retina Society, Professor Michael Stewart of the Mayo Clinic in Jacksonville, Florida, US, and Professor Voraporn Chaikitmongkol of Chiang Mai University in Chiang Mai, Thailand, shared real-world experiences supporting the role of aflibercept in management of neovascular age-related macular degeneration (nAMD) and polypoidal choroidal vasculopathy (PCV).
Anti-VEGFs as SoC in nAMD and PCV
“Exudative retinal diseases result primarily from excessive activation of the VEGF receptor [VEGFR]-1/2 pathway, which is considered the master regulator of pathological angiogenesis in these conditions,” said Stewart. [Prog Retin Eye Res 2021;84:100954; Nat Rev Drug Discov 2017;16:635-661; Angiogenesis 2012;15:171-185]
This is why anti-VEGFs have emerged as standard of care (SoC) for several exudative retinal diseases, including nAMD and PCV. [Ophthalmology 2020;127:P1-P65; Asia Pac J Ophthalmol (Phila) 2021;10:507-518] Among the available anti-VEGFs, aflibercept is known to bind to the most diverse range of VEGFR-1/2 ligands (ie, VEGF-A, VEGF-B, placental growth factor and galectin-1) that are considered key drivers of retinal diseases. [Prog Retin Eye Res 2021;84:100954; Sci Rep 2015;5:17946]
ARIES: Continuous proactive aflibercept T&E regimen
Several trials have demonstrated the efficacy and safety of a treat-and-extend (T&E) regimen with aflibercept, which allows flexibility of injection intervals of up to Q16W while achieving and maintaining vision gains. [Adv Ther 2020;37:1173-1187; Retina 2021;41:1911-1920; Ophthalmol Ther 2022;11:613-627]
The randomized, phase IIIb/IV, open-label ARIES study (n=271) produced similar outcomes between treatment-naive nAMD patients who received early- and late-start aflibercept T&E regimens. [Retina 2021;41:1911-1920]
A post hoc analysis of ARIES investigated outcomes in patients with treatment-naive nAMD receiving T&E aflibercept for 104 weeks, who met criteria for an early hypothetical switch to another anti-VEGF agent (eg, presence of central intraretinal and/or subretinal fluid [IRF/SRF] at week 8 or 24, gains in best-corrected visual acuity [BCVA] ≤5 letters). It transpired that BCVA outcomes were not worse in hypothetical switchers who in fact continued aflibercept treatment compared with non-switchers. For instance, in patients with vs without central IRF/SRF at week 24, mean change in BCVA at week 104 was +5.7 vs +3.4 letters. “There is thus little rationale for early switching from continuous proactive aflibercept T&E regimen in these patients,” commented Stewart. [Ophthalmol Ther 2022;11:613-627]
In another post hoc analysis of ARIES, up to 23 percent of patients were identified as requiring injection-intensive aflibercept dosing. These were patients who needed a treatment interval of <Q8W and had ≥1 interval of <Q8W dosing after three initial monthly doses of aflibercept. These patients experienced improvements in vision and anatomic outcomes with continued aflibercept treatment, and most could still be extended to ≥Q8W and even up to Q16W intervals after being on a <Q8W regimen. [Ophthalmol Ther 2022;11:1793-1803]
“These results highlight the importance of treatment continuity and an individualized T&E approach for all patients with nAMD, including those who require an injection-intensive regimen or who are hypothetical switchers [eg, patients with residual SRF], to achieve clinically meaningful vision gains with aflibercept,” stressed Stewart. [Ophthalmol Ther 2022;11:613-627; Ophthalmol Ther 2022;11:1793-1803]
Aflibercept T&E in PCV
A multicentre, prospective, nonrandomized, interventional study (n=97) in Japan reported that aflibercept injections with a 1-month–adjusted T&E regimen significantly improved BCVA and central retinal thickness (CRT) with a reduced number of injections at 2 years in patients with PCV and nAMD. Patients with PCV and nAMD maintained vision gains at year 2 with aflibercept, with 67.3 percent of PCV patients being maintained on Q12W dosing intervals. [Ophthalmol Retina 2020;4:767-776]
Outcomes comparable to randomized controlled trials (RCTs) were demonstrated in real-world PCV patients in several retrospective case series. [Jpn J Ophthalmol 2017;61:150-158; Acta Med Okayama 2018;72:379-385; Graefes Arch Clin Exp Ophthalmol 2017;255:1891-1897; Ophthalmol Ther 2020;9:1069-1082]
Continuing aflibercept after complete polypoidal regression
“We conducted an interventional study in 79 patients with treatment-naive PCV in Thailand to determine the interval of disease inactivity as per optical coherence tomography [OCT] following complete polypoidal regression after aflibercept monotherapy,” said Chaikitmongkol. Treatments were deferred in patients with complete polypoidal regression after initial 3- to 6-monthly aflibercept injections, while those with partial or no regression after six doses continued on a T&E aflibercept regimen. [NCT04707027; Chaikitmongkol V, et al, unpublished]
Preliminary results at 1-year follow-up showed that 53 percent of the eyes gained ≥15 letters from baseline. At the same time, after 3–6 aflibercept injections, 32 of 79 eyes (41 percent) that achieved complete polypoidal regression had exudative recurrence after treatment deferrals at 1 year. Median time to exudative recurrence was 5 months (interquartile range, 4–5 months) after the last injection. [Chaikitmongkol V, et al, unpublished]
“These findings indicate that early discontinuation of aflibercept treatment may lead to exudative recurrence, which frequently occurred <6 months after treatment deferrals, even in PCV eyes that had previously achieved complete polypoidal regression [meaning that early discontinuation of aflibercept treatment is not recommended],” said Chaikitmongkol.
Aflibercept’s real-world outcomes reflective of RCTs
“Aflibercept has been delivering SoC outcomes for over a decade, with robust data from long-term follow-up studies supporting its role as a first-line option for various retinal disease indications, including nAMD and PCV,” said Stewart. “Encouragingly, its RCT data have been reflected in long-term real-world outcomes.”
In a retrospective, single-centre, nonrandomized interventional cohort analysis of 94 eyes of 89 patients with nAMD in the UK who had completed 4-year follow-up, the mean number of aflibercept injections received over 4 years was 19.3. Mean change in ETDRS letters was +6.3, while 33 percent of the eyes gained ≥15 ETDRS letters at the end of 4 years. [Eur J Ophthalmol 2021;31:1940-1944]
“Real-world studies in patients with PCV treated with T&E aflibercept [also] demonstrated results comparable with RCTs,” noted Chaikitmongkol. For instance, in an analysis of 37 eyes with treatment-naive PCV, which received aflibercept T&E regimen, the minimum angle of resolution BCVA improved from 0.37 at baseline to 0.21 at 1 year (p<0.001), while the mean CRT decreased from 342.3 μm at baseline to 196.6 μm at 1 year (p<0.001). The mean treatment interval was 12 weeks in 59.5 percent of the eyes. [Jpn J Ophthalmol 2017;61:150-158] Following 2 years of treatment, the mean total number of injections in the 2-year period in the eyes with polyp regression after the loading phase (PR+; n=19) was 12.4 vs 15.3 in eyes without polyp regression after the loading phase (PR-; n=18). The mean intervals between injections at year 2 in the PR+ and PR- groups were 12.5 weeks and 9.6 weeks, respectively. At 2 years, the injection interval could be extended to Q16W in 47.3 percent of PR+ eyes. [Acta Med Okayama 2018;72:379-385]
In a retrospective interventional case series of 58 eyes of 58 Japanese patients with PCV treated with T&E aflibercept, the number of injections averaged 7.72 in year 1 and 4.67 in year 2. The average number of polypoidal lesions per patient was 2.43 before treatment. In 55.2 percent of patients, polypoidal lesions regressed completely after the loading phase, and these patients needed significantly fewer injections vs other patients. Central macular thickness and central corneal thickness (CCT) significantly reduced and were maintained throughout the follow-up period, while BCVA significantly improved after the loading phase and was maintained in the maintenance phase. [Graefes Arch Clin Exp Ophthalmol 2017;255:1891-1897]
According to the Asia-Pacific Vitreo-Retina Society, vision gains may still be achieved with a T&E regimen without the need to aim for complete fluid resolution. [Asia Pac J Ophthalmol (Phila) 2021;10:507-518] In ARIES, for instance, patients were still allowed to continue with a T&E regimen despite having residual SRF, ie, thickness ≤50 μm. [Retina 2021;41:1911-1920]
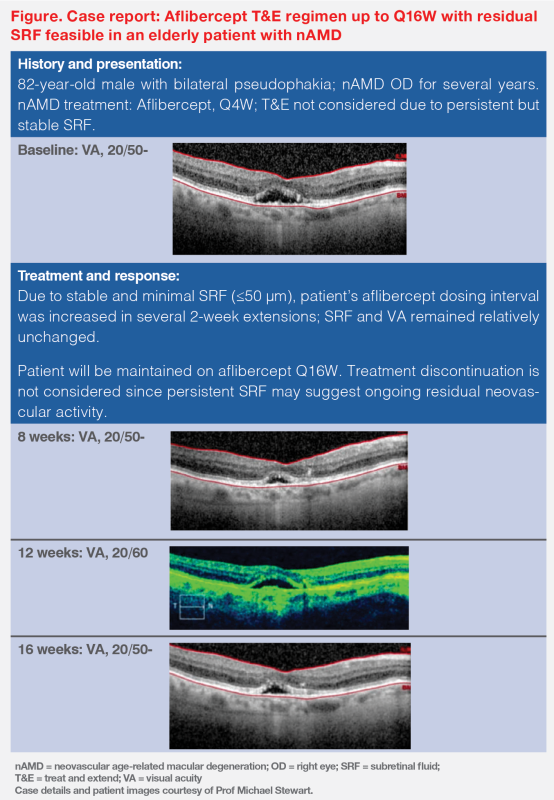
“This is consistent with what we’ve seen in clinical practice,” said Stewart. “We demonstrated in an elderly patient with nAMD that a T&E regimen with up to Q16W intervals was feasible with aflibercept without requiring a completely dry retina as long as the SRF was minimal and stable.” (Figure)
Does switching from aflibercept to faricimab work?
“Anytime a new drug becomes available, we tend to ask if switching therapies will be beneficial, especially if results with current treatments have been suboptimal,” said Stewart. “With the monoclonal antibody, faricimab, however, we’ve noticed a mixed bag of results in patients with nAMD.”
A retrospective study evaluated 55 eyes from 55 Japanese nAMD patients who received aflibercept every <Q8W and were switched to faricimab. After three injections of faricimab, the BCVA and CCT did not change significantly, and 71 percent of eyes still required <Q8W interval injections. [J Clin Med 2023;12:5145]
A multicentre retrospective study in Japan examined 130 eyes with nAMD from 124 patients receiving monthly aflibercept, which were switched to faricimab administered monthly for ≤4 injections followed by ≥Q8W interval injections. At 6 months, 59.2 percent of eyes (n=77) discontinued faricimab for various reasons, including insufficient efficacy in 92.2 percent of cases (n=71). Among those, 78.9 percent (n=56) switched back to aflibercept. [Graefes Arch Clin Exp Ophthalmol 2024;262:43-51]
Aflibercept’s real-world safety
A search of aflibercept’s Global Safety Pharmacovigilance Database between October 2012 and March 2022 reported low rates of reported cases of intraocular inflammation (IOI), including endophthalmitis. [Clin Ophthalmol 2023;17:385-390]
With >10 years of postmarketing experience with aflibercept vials (>25 million sold units) and >2 years of experience with aflibercept prefilled syringes (PFS; >6.7 million sold units), the rate of any IOI, including endophthalmitis, outside the US, was 0.3 events per 10,000 units for PFS and 1.2 events per 10,000 units for aflibercept in vials. Endophthalmitis occurred at a rate of 1 in 16,000 injections when aflibercept was administered from a vial and was approximately 1 in 100,000 intravitreal injections when administered via the PFS.