Multidisciplinary management of a patient with CRSwNP and comorbid asthma using add-on anti–IL-5









History and initial treatment
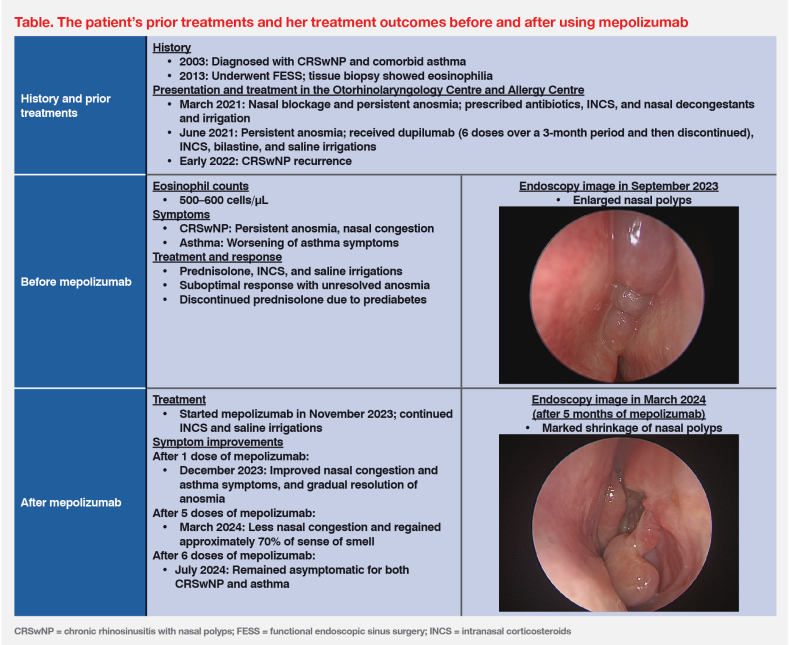
A 60-year-old female nonsmoker was first diagnosed with chronic rhinosinusitis with nasal polyps (CRSwNP) and comorbid asthma in 2003. (Table) Due to anosmia (loss of sense of smell), nasal congestion and postnasal drip, she underwent functional endoscopic sinus surgery (FESS) in 2013, which generally improved her symptoms. Tissue biopsy showed abundant eosinophils in nasal polyps. She used daily puffs of a budesonide/formoterol inhaler for asthma control. (Table)
Presentation, subsequent treatments and response
In March 2021, the patient presented at the Li Shu Pui ENT Head & Neck Surgery Centre (Otorhinolaryngology Centre) with nasal blockage and persistent anosmia. Endoscopic findings confirmed recurrence of bilateral nasal polyps. She was treated with antibiotics, antihistamines, intranasal corticosteroids (INCS), nasal decongestants, and nasal irrigation. (Table) After 2–3 weeks, her nasal obstruction improved, while anosmia remained.
In view of persistent anosmia, the patient was referred to the Lee Tak Hong Allergy Centre (Allergy Centre) in April 2021. In June 2021, she started biologic treatment with dupilumab 300 mg subcutaneous (SC) injections Q2W, which she received for 3 months for a total of six doses. Concomitant medications included INCS, bilastine and saline nasal wash. Although the patient’s symptoms gradually improved, the treatment was discontinued due to financial constraints. She continued to receive maintenance treatment with saline irrigations. (Table)
In early 2022, less than 6 months since discontinuing dupilumab, the patient experienced nasal polyp recurrence with worsening CRSwNP symptoms, including anosmia, nasal congestion, and worsening of asthma symptoms. In September 2023, endoscopy showed enlarged nasal polyps. With the polyp and anosmia recurrence, the patient was again referred to the Allergy Centre in November 2023. (Table)
Laboratory examinations upon referral showed elevated eosinophil count (500–600 cells/μL). Skin prick test revealed severe dust mite allergy. These findings reaffirmed recurrent CRSwNP with T2 inflammatory signatures. The patient initially received prednisolone (25 mg/day) and INCS therapy, which resulted in only partial improvements in CRSwNP and asthma symptoms. Her anosmia did not resolve. After 2 weeks, prednisolone was discontinued since she developed prediabetes. (Table)
The patient expressed particular concern about persistent anosmia. After discussing the latest available treatment options, she agreed to start therapy with another biologic agent, the anti–interleukin (IL)-5 monoclonal antibody (mAb), mepolizumab 100 mg SC injections Q4W in late November 2023, as add-on treatment to INCS and PRN saline irrigations. After the first dose, the patient noted early improvements in symptoms, including less nasal congestion and gradual resolution of anosmia. Asthma symptoms also improved. (Table)
In March 2024, before receiving the fifth dose of mepolizumab, endoscopy confirmed marked shrinkage of nasal polyps. (Table) Importantly, the patient regained approximately 70 percent of her sense of smell. She received six doses of mepolizumab and was recommended to continue the therapy. (Table)
During the treatment period, the patient did not experience adverse events (AEs) related to mepolizumab. Last seen in July 2024, she was well and remained asymptomatic for both CRSwNP and asthma. (Table)
Discussion
CRSwNP is linked to type 2 inflammation, which tends to be persistent and recurrent despite standard-of-care (SoC) treatment with INCS, saline irrigations and short courses of systemic corticosteroids (SCS).1-4 FESS is considered when CRSwNP is refractory to SoC.1,3 However, recurrence of nasal polyps after FESS is common. In general, >50 percent of patients may experience recurrence at 3–12 years after surgery.4-7 This is similar to our clinical experience, where recurrence may occur in up to half of our patients with CRSwNP. In particular, approximately 40 percent of those who undergo surgery will experience recurrence within 1–2 years. Notably, up to 60 percent of patients prefer pharmacological treatments over surgery, especially those with comorbidities.
As experienced by our patient, anosmia is one of the most bothersome symptoms of CRSwNP, particularly because it diminished her sense of safety in daily life. Literature shows that quality of life (QoL) is negatively impacted (eg, difficulties with cooking, taste loss, feeling less safe, depression, and anxiety).8,9 In CRSwNP, olfactory dysfunction may result from mechanical obstruction, increased inflammatory mediators in the nose and paranasal sinuses, or both, making it a sensitive indicator of inflammatory burden and disease severity.1,9
However, repeated surgeries for nasal polyp recurrence can be burdensome. Of note, 50 percent of our patients report no olfactory function improvement after surgery. Long-term SCS use is associated with multiple side effects, prompting the development of biologic therapies targeting different aspects of the T2 inflammatory pathway.2
In particular, the T2 inflammatory cytokine, IL-5, has become an important target due to its critical role in pathogenesis of eosinophilic CRS and nasal polyp growth.10,11 Mepolizumab, an anti–IL-5 mAb, is selective and effective in inhibiting eosinophilic inflammation. Since IL-5 is a major cytokine involved in the growth, differentiation, recruitment, activation, and survival of eosinophils, inhibiting IL-5 signalling reduces eosinophil production and survival, effectively inhibiting eosinophilic inflammation. Mepolizumab is approved both as an add-on maintenance treatment for patients with severe eosinophilic asthma and as an add-on therapy with INCS for adults with severe CRSwNP for whom SCS and/or surgery do not provide adequate disease control.12
Robust evidence also demonstrates mepolizumab’s efficacy in reducing nasal polyp size and nasal obstruction symptoms, thus reducing or delaying the need for surgery and improving QoL.2,4,13 Early improvement of anosmia symptoms was also observed with mepolizumab in patients with CRSwNP, which may address the inflammatory aetiology of olfactory symptoms.1,9
As demonstrated in our patient’s case, CRSwNP, anosmia and comorbid asthma are due to the common underlying T2 inflammation, as indicated by high blood eosinophil level. Eosinophilia is a critical biomarker of T2 inflammation. 14 Based on our experience, currently approved biologics are effective for CRSwNP with comorbid asthma, but mepolizumab is preferable when blood or tissue eosinophil levels are high. Therefore, mepolizumab appeared to be a suitable option for our patient’s condition.12,14 This was confirmed by her early and sustained response to mepolizumab.
Since informed consent regarding biologic use is crucial, patients must understand why biologic agents are being considered. This includes explaining the nature of their disease (ie, CRSwNP pathogenesis), the role of T2 inflammation, including the roles of T2 cytokines and eosinophils, and why SoC and surgery may be insufficient. In our patient’s case, we explained that anti–IL-5 treatment could improve her concurrent asthma and help restore her sense of smell.
Our patient agreed to start biologic therapy after experiencing CRSwNP recurrence with persistent anosmia despite SoC approaches. She initially used dupilumab for 3 months, but soon experienced disease recurrence <6 months after treatment discontinuation. Due to concerns about persistent anosmia and worsening asthma symptoms after the recurrence, mepolizumab was started last year with a committed treatment period of 6 months, as recommended in the European Position Paper on Rhinosinusitis and Nasal Polyps (EPOS) guidelines, to assess treatment response.4 Of note, those with at least an initial partial response may still improve with continued mepolizumab treatment beyond 6 months.12 Since our patient was an early responder, continued mepolizumab treatment was recommended after 6 months to maintain the improvement of her CRSwNP symptoms.
Based on our experience, surgery and biologics can complement each other rather than being mutually exclusive. One of our patients initially consulted us for different treatment options. He started with mepolizumab and noticed significant improvement after just 2–3 doses. After 7 doses, he decided to undergo surgery for full nasal passage clearance, and he was willing to continue mepolizumab to prevent CRSwNP recurrence after the surgery.
Our patient’s experience highlights the importance of a multidisciplinary approach in CRSwNP management. Coordination between the otorhinolaryngologist and allergy specialist can help patients understand their condition, assess treatment options, and manage treatment response expectations, thereby providing a comprehensive solution. Our case, along with currently available evidence, supports mepolizumab as an effective and safe add-on treatment for those with CRSwNP with comorbidities such as asthma.4,11,13