
The treatment landscape of metastatic renal cell carcinoma (mRCC) has undergone a major transformation in the past two decades, with interleukin-2 and interferon-α being replaced with anti-angiogenic tyrosine kinase inhibitors (TKIs), inhibitors of mammalian target of rapamycin (mTOR) and immune checkpoint inhibitors (ICIs). At an Eisai-sponsored symposium organized by the Hong Kong Society of Uro-Oncology during Uro-Oncology Asia 2024, Professor Sun-Young Rha of the Yonsei University College of Medicine in Seoul, South Korea, discussed the importance of optimizing first-line treatment and subsequent strategies for patients with advanced RCC. Rha also reviewed clinical evidence on regimens containing lenvatinib (Lenvima®, Eisai), an oral TKI against VEGF receptor, which have been shown to improve outcomes in first and subsequent lines of therapy.
“Systemic treatment for mRCC has been evolving over the last two decades,” said Rha. “After cytokine-based therapies, came the first two targeted TKIs, namely, sorafenib and sunitinib [in 2005 and 2006, respectively]. Since then, >10 targeted agents and ICIs have been developed for first- and subsequent-line use.” [Cancers (Basel) 2023;15:665; National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology, Kidney Cancer, version 2.2024]
Optimizing first-line treatment for mRCC
“Optimizing first-line treatment in mRCC is important because we lose many eligible patients in succeeding lines of therapy,” commented Rha. “For instance, a retrospective study by the International mRCC Database Consortium [IMDC] showed that among patients [n=7,498] who received first-line mRCC treatment, only 51.4 percent [n=3,854] moved on to receive second-line therapy.” [Kidney Cancer 2018;2:31-36]
Improving clinical outcomes with first-line therapies
“Since their introduction into first-line treatment armamentarium, immuno-oncology [IO]–based regimens targeting different pathways [eg, immunoregulatory pathways, pro-angiogenic pathways] have markedly improved outcomes of mRCC patients,” noted Rha. [Cancers (Basel) 2023;15:665; NCCN Clinical Practice Guidelines in Oncology, Kidney Cancer, version 2.2024]
The IO-IO combination of nivolumab (an anti–PD-1 antibody) plus ipilimumab (an anti–CTLA-4 antibody), and IO-TKI combinations (eg, nivolumab plus cabozantinib [a multitargeted TKI], or pembrolizumab [an anti–PD-1 antibody] plus axitinib [an anti–VEGF receptor TKI] or lenvatinib [a multitargeted TKI]) have shown especially promising results. [Ther Adv Med Oncol 2022;14:1-17]
“Although IO-based regimens’ efficacy and safety have been demonstrated in clinical trials, selecting optimal IO combinations in the first-line setting remains a challenge,” commented Rha. Since it is not feasible to directly compare results of individual trials, real-world data may offer additional insights into outcomes of patients treated with different combinations.
Real-world ARON-1 study of IO-TKI vs IO-IO combos
ARON-1 was a retrospective, global real-world study evaluating first-line IO combinations in patients with mRCC. Of 729 adult patients (median age, 63 years; male, 74 percent; clear-cell RCC [ccRCC], 86 percent), 313 received IO-IO therapy, while 416 received IO-TKI combinations. At a median follow-up of 18.1 months, median overall survival (OS) in the overall study population was 36.5 months, while median progression-free survival (PFS) was 15.0 months. [Target Oncol 2023;18:559-570]
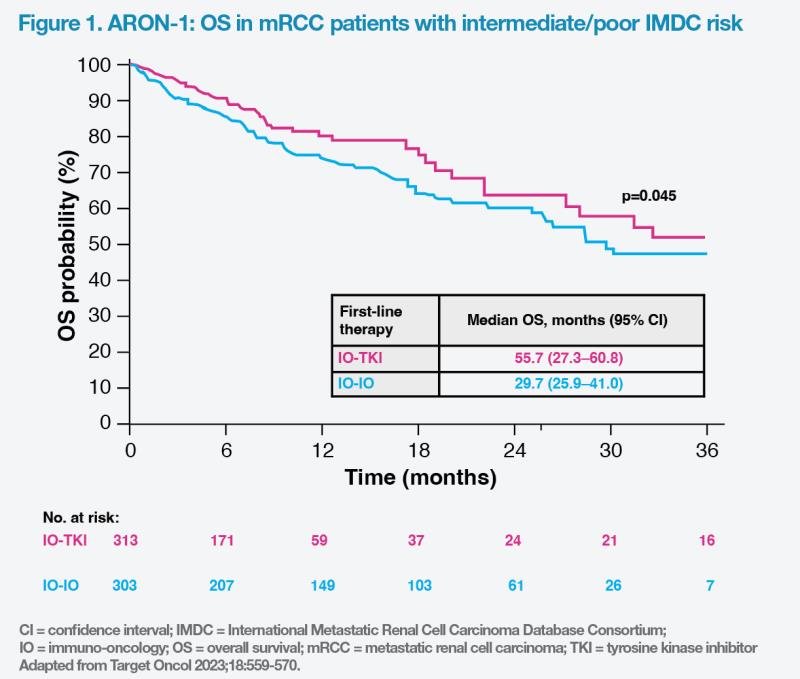
Among 616 patients with intermediate/poor IMDC risk, the IO-TKI combination group reached significantly longer median OS vs the IO-IO combination group (55.7 months vs 29.7 months; p=0.045). (Figure 1) Median PFS was also significantly longer in the IO-TKI vs IO-IO combination group (15.9 months vs 11.1 months; p=0.011).
“We can expect better survival outcomes with IO-TKI vs IO-IO combinations [as first-line therapy for mRCC patients],” commented Rha.
First-line lenvatinib plus pembrolizumab in advanced RCC: Phase III CLEAR trial
In US NCCN’s kidney cancer treatment guidelines, first-line TKI-IO combination with lenvatinib and pembrolizumab is recommended as a category 1, preferred treatment option for patients with advanced ccRCC across all risk groups. [NCCN Clinical Practice Guidelines in Oncology, Kidney Cancer, version 2.2024]
This recommendation is based on results of the phase III CLEAR trial, which included 1,069 adult patients with advanced ccRCC with ≥1 measurable lesion who had not previously received systemic therapy. The patients were randomized to receive lenvatinib (20 mg orally, QD) plus pembrolizumab (200 mg intravenously, Q3W), lenvatinib (18 mg orally, QD) plus everolimus (5 mg orally, QD), or sunitinib monotherapy (50 mg orally QD, 4 weeks on, 2 weeks off). Primary analysis results showed that only lenvatinib plus pembrolizumab was associated with significant improvement in both PFS (hazard ratio [HR], 0.39; 95 percent CI, 0.32–0.49; p<0.001) and OS (HR, 0.66; 95 percent CI, 0.49–0.88; p=0.005) vs sunitinib, at a median follow-up for OS of 26.6 months (data cut-off, 28 August 2020). [NCCN Clinical Practice Guidelines in Oncology, Kidney Cancer, version 2.2024; N Engl J Med 2021;384:1289-1300]
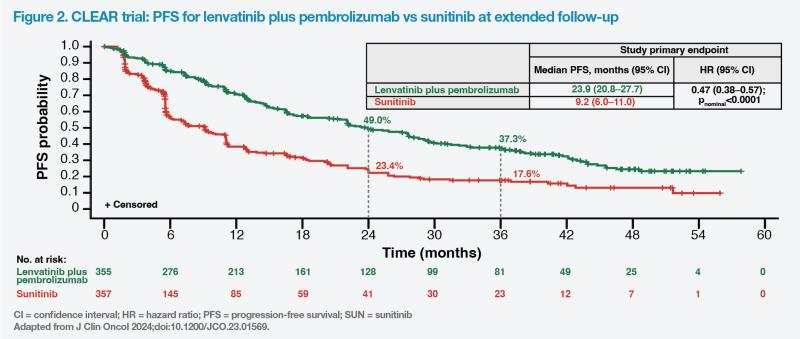
After an additional follow-up of 23 months from the primary analysis, median PFS benefit continued to be observed for lenvatinib plus pembrolizumab vs sunitinib (23.9 months vs 9.2 months; HR, 0.47; 95 percent CI, 0.38– 0.57; p<0.0001). (Figure 2) [J Clin Oncol 2024;doi:10.1200/JCO.23.01569]
Final prespecified OS analysis showed clinically meaningful efficacy with lenvatinib plus pembrolizumab vs sunitinib (median OS, 53.7 months vs 54.3 months; HR, 0.79; 95 percent CI, 0.63–0.99; pnominal=0.0424). Corresponding OS rates at 36 months were 66.4 percent vs 60.2 percent.
According to Rha, long-term OS observed in the treatment groups could be affected by the use of subsequent therapy. “In the CLEAR trial, fewer patients in the lenvatinib plus pembrolizumab vs sunitinib group received subsequent anticancer therapies [51.0 percent vs 68.9 percent], including ICIs [15.8 percent vs 54.6 percent]. The adjusted HR for death was 0.55 [95 percent CI, 0.44– 0.69],” she said.
“Other notable results in favour of lenvatinib plus pembrolizumab vs sunitinib were objective response rate [ORR; 71.3 percent vs 36.7 percent; relative risk, 1.94] and complete response rate [18.3 percent vs 4.8 percent], demonstrating sustained deep responses with the IO-TKI combination,” added Rha.
Safety results from CLEAR were consistent with the established safety profiles of each agent and of the combination in patients with other solid tumours. Grade ≥3 treatment-emergent adverse events (TEAEs) occurred in 84.9 percent of patients treated with lenvatinib plus pembrolizumab and in 74.7 percent of patients treated with sunitinib. In the lenvatinib plus pembrolizumab group, the most common grade ≥3 TEAEs included hypertension (29.0 percent), proteinuria (9.9 percent) and diarrhoea (11.1 percent). “These AEs depend on the patient’s condition and may differ among patients. Dose modification is important for managing toxicities,” Rha said.
“After 4 years of follow-up, sustained survival and durable response benefits were observed with first-line lenvatinib plus pembrolizumab vs sunitinib in patients with previously untreated advanced RCC,” she added.
Salvage therapy for mRCC
“To date, multiple salvage treatments have become available for patients with ccRCC progressing after systemic therapy,” Rha noted. [Lancet 2008;372:449- 456; Cancer 2010;116:4256-4265; Lancet Oncol 2013;14:552-562]
For patients with high tumour burden and rapidly progressing disease who received a prior VEGF TKI, a phase II, randomized, open-label, multicentre trial demonstrated a median PFS by independent radiological review of 12.8 months in the lenvatinib plus everolimus group vs 5.6 months in the everolimus alone group (HR, 0.45; 95 percent CI, 0.27–0.79; p=0.0029). [Lancet Oncol 2016;17:e4-e5] In addition, significantly more patients who received lenvatinib plus everolimus vs those who received everolimus monotherapy achieved an objective response (43 percent vs 6 percent; rate ratio, 7.2; p<0.0001). [Lancet Oncol 2015;16:1473-1482]
Phase III trials showed better ORR and PFS with second-line cabozantinib vs everolimus and second-line nivolumab vs everolimus at extended follow-up. “Lenvatinib plus everolimus is a preferred second-line option for ccRCC,” Rha commented. [Lancet Oncol 2016;17:917-927; Cancer 2020;126:4156-4167; Lancet Oncol 2015;16:1473-1482]
“Despite insufficient data regarding subsequent treatment [sequencing] after IO therapies, a retrospective study showed longer OS and higher ORR with VEGF TKIs vs mTOR inhibitors in patients with IO-pretreated mRCC,” she noted. [NCCN Clinical Practice Guidelines in Oncology, Kidney Cancer, version 2.2024; Eur Urol Oncol 2021;4:102-111]
In a recent single-arm, phase II study, second-line cabozantinib demonstrated a median PFS of 8.3 months in IO-pretreated mRCC patients. However, adding atezolizumab to cabozantinib in a phase III trial did not improve clinical outcomes and led to increased toxicity in mRCC patients who had received first-line IO treatment. [Tumori 2023;109:129- 137; Lancet 2023;402:185-195] “TKIs remain important [in the second-line setting],” Rha added.
Conclusion
“The treatment landscape for advanced RCC is rapidly evolving with emergence of novel agents as well as unique combination strategies [eg, lenvatinib plus pembrolizumab],” reiterated Rha. “[By targeting both pro-angiogenic and immunoregulatory pathways,] first-line lenvatinib plus pembrolizumab demonstrated clinically meaningful and durable efficacy as well as manageable safety profile vs sunitinib in advanced RCC patients.”