Metastatic colorectal cancer (mCRC) is one of the most malignant diseases with a poor prognosis. In an interview with MIMS Oncology, Dr Ingrid Karmane Sumou, a medical oncologist in Macau, shared several patient cases and discussed treatment strategies, including the use of regorafenib, to improve survival outcomes and provided tips on managing regorafenib-related adverse effects.
Longer survival with more treatment lines
“Recent clinical trials of first-line therapies for mCRC have shown median overall survival [mOS] of approximately 30 months,” Sumou said. [Cancer Treat Rev 2017:59:54-60]
The increase in treatment options in late-line settings has extended patients’ median OS. A retrospective study in mCRC patients (n=1,409) revealed that those who received multiple lines of treatment, including late-line regorafenib or trifluridine/ tipiracil (TAS-102), had prolonged mOS of >35 months. “Therefore, as oncologists, we always strive to maximize patients’ exposure to as many treatment lines as possible to improve survival outcomes,” Sumou stressed. [Cancer Med 2022;11:2184-2192]
Early-line treatment options
“In our hospital, upfront tissue-based next-generation sequencing sequencing [NGS] is often performed for patients who present with de novo mCRC,” Sumou shared. “Treatment choices are personalized based on patients’ gene mutations, microsatellite instability [MSI] status, Eastern Cooperative Oncology Group performance status [ECOG PS], and personal preference.”
First- and second-line treatment options include fluorouracil-based chemotherapy and VEGF inhibitor–based therapy (mainly bevacizumab). For patients with left-sided RAS wild-type tumours, EGFR-targeted therapies, such as cetuximab and panitumumab, can be used. Immune checkpoint inhibitor therapy is indicated for patients with MSI-high disease (prevalence, 5 percent). [NCCN Guidelines, Colon Cancer, version 2.2024; NCCN Guidelines, Rectal Cancer, version 2.2024; Front Oncol 2022;12:888181]
Treatment options for refractory mCRC
“Patients with disease progression after first- and second-line therapy can be rechallenged with a previous therapy. In individual cases, we may perform a liquid biopsy to reconfirm RAS status and evaluate suitability of EGFR-targeted therapy,” said Sumou. “Regorafenib, fruquintinib, and TAS-102 plus bevacizumab are targeted drug options that can be used in the third line and beyond.”
Oral multikinase inhibitor
Regorafenib targets the activity of several protein kinases involved in regulation of tumour angiogenesis (VEGF receptors 1–3 and TIE2), oncogenesis (KIT, RET, RAF1, and BRAF), and the tumour microenvironment (platelet-derived growth factor and fibroblast growth factor receptor [FGFR]). [Stivarga Hong Kong Prescribing Information, November 2019]
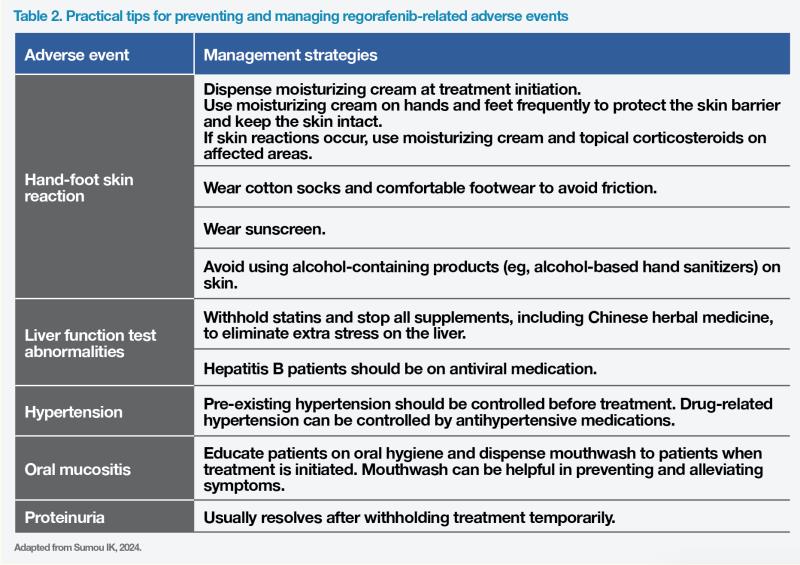
In the phase III CORRECT trial, regorafenib significantly prolonged mOS (6.4 vs 5.0 months; hazard ratio [HR], 0.77; 95 percent confidence interval [CI], 0.64–0.94; p=0.0052) and median progression-free survival (mPFS; 1.9 vs 1.7 months; HR, 0.49; 95 percent CI, 0.42–0.58; p<0.0001) vs placebo in previously treated mCRC patients. (Table 1) [Lancet 2013;381:303-312]
Regorafenib demonstrated even greater survival benefits in the Asian population, including patients from mainland China, Hong Kong, South Korea, Taiwan, and Vietnam, in the phase III CONCUR trial than in the global population of CORRECT. Among Asian patients in CONCUR, mOS was 8.8 vs 6.3 months for regorafenib vs placebo (HR, 0.55; 95 percent CI, 0.40–0.77; p=0.00016), while mPFS was 3.2 vs 1.7 months (HR, 0.31; 95 percent CI, 0.22–0.44; p<0.0001), after a median follow-up of 7.4 months. (Table 1) [Lancet Oncol 2015;16:619-629]
Consistent with other multikinase inhibitors, one of the most common adverse events (AEs) associated with regorafenib is hand-foot skin reaction (HFSR). Other common AEs include fatigue, hepatic derangement, and hypertension. [Lancet Oncol 2015;16:619-629]
Oral VEGF receptor inhibitor
Fruquintinib is another therapy approved for mCRC patients with disease progression after receiving ≥2 lines of systemic therapy. The phase III FRESCO trial conducted in patients with refractory mCRC in mainland China showed significantly prolonged mOS (9.3 vs 6.6 months; HR, 0.65; 95 percent CI, 0.51–0.83; p<0.001) and mPFS (3.7 vs 1.8 months; HR, 0.26; 95 percent CI; 0.21–0.34; p<0.001) with fruquintinib vs placebo after a median follow-up of 13 months. Fruquintinib also significantly improved mOS (7.4 vs 4.8 months; HR, 0.66; 95 percent CI, 0.55–0.80; p<0.0001) and mPFS (3.7 vs 1.8 months; HR, 0.32; 95 percent CI, 0.27–0.39; p<0.0001) vs placebo in the international phase III FRESCO-2 trial after a median follow-up of 11 months. (Table 1) HFSR, hypertension, and asthenia were the most common AEs leading to dose reduction, whereas asthenia was the most common AE leading to fruquintinib discontinuation. [JAMA 2018;319:2486-2496; Lancet 2023;402:41-53]
Oral chemotherapy + intravenous bevacizumab
In the recent phase III SUNLIGHT trial, continuous inhibition of angiogenesis with bevacizumab plus TAS-102 chemotherapy in the third-line setting demonstrated efficacy in prolonging mOS (10.8 vs 7.5 months; HR, 0.61; 95 percent CI, 0.49–0.77; p<0.001) and mPFS (5.6 vs 2.4 months; HR, 0.44; 95 percent CI, 0.36–0.54; p<0.001) vs TAS-102 alone, after a median follow-up of 14 months, in patients with refractory mCRC who had disease progression following ≤2 previous chemotherapy regimens. (Table 1) However, grade ≥3 AEs were reported in the majority of patients (72 vs 70 percent for bevacizumab plus TAS- 102 vs TAS-102 alone), serious AEs were reported in 25 vs 31 percent of the patients, and AEs led to treatment discontinuation in 13 percent of patients in each group. [N Engl J Med 2023;388:1657-1667]
Importance of treatment sequence
“Although increasing the number of treatment lines may prolong survival, patients often experience gradual worsening of ECOG PS from both disease progression and the cumulative AEs of each treatment. Patients with poor ECOG PS tend to have difficulty tolerating rigorous treatment and have less favourable outcomes. Therefore, we tend to use more rigorous treatments, such as regorafenib or chemotherapy rechallenge, at earlier lines when patients are still fit after receiving two lines of systemic treatment, and reserve milder treatments, such as fruquintinib, for later lines,” pointed out Sumou. “Rechallenge is particularly useful when a patient had durable and deep response to prior chemotherapy or targeted agents.”
As illustrated in case 1, the patient initially responded to and tolerated chemotherapy well, and thus was rechallenged with chemotherapy in the third line before trying anti-VEGF therapy. He then received regorafenib while he was still fit, followed by fruquintinib and TAS-102 plus bevacizumab, which was just approved at that time. Careful sequencing of ≥7 treatment lines has produced a very long and still ongoing OS of ≥78 months.

“Of note, this intravenous regimen of TAS-102 plus bevacizumab is less convenient to administer than oral regimens, entails frequent hospital visits and is healthcare resource–intensive. Tolerability is also a concern, especially in patients who could not tolerate prior chemotherapy. As such, TAS-102 plus bevacizumab is reserved for fit patients with good supportive care who are still willing to try chemotherapy,” noted Sumou. [N Engl J Med 2023;388:1657- 1667]
“We generally favour the use of regorafenib vs fruquintinib as third-line therapy because later-line initiation of regorafenib may not be feasible when patients become frail,” explained Sumou. “In addition, real-world evidence demonstrates that using regorafenib before fruquintinib prolongs OS, which is the ‘gold standard’ endpoint in cancer treatment.”
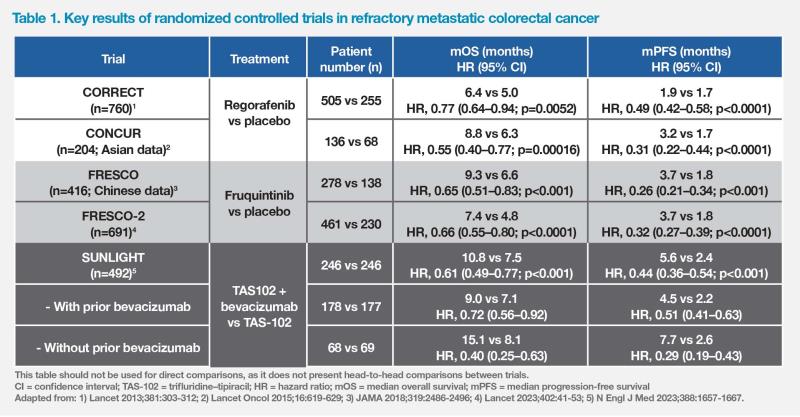
In a treatment sequence analysis of an observational cohort study conducted in Beijing Cancer Hospital, China, patients who received regorafenib followed by fruquintinib had significantly longer OS than those who received the reverse sequence (28.1 vs 18.4 months; p=0.024). (Figure 1) A real-world retrospective study conducted in Zhejiang Provincial People’s Hospital, China, also demonstrated significantly longer OS in patients treated with regorafenib followed by fruquintinib vs the reverse sequence (15.0 vs 8.3 months; p=0.019). [Clin Colorectal Cancer 2022;21:e152-e161; Front Oncol 2023:13:1097911]
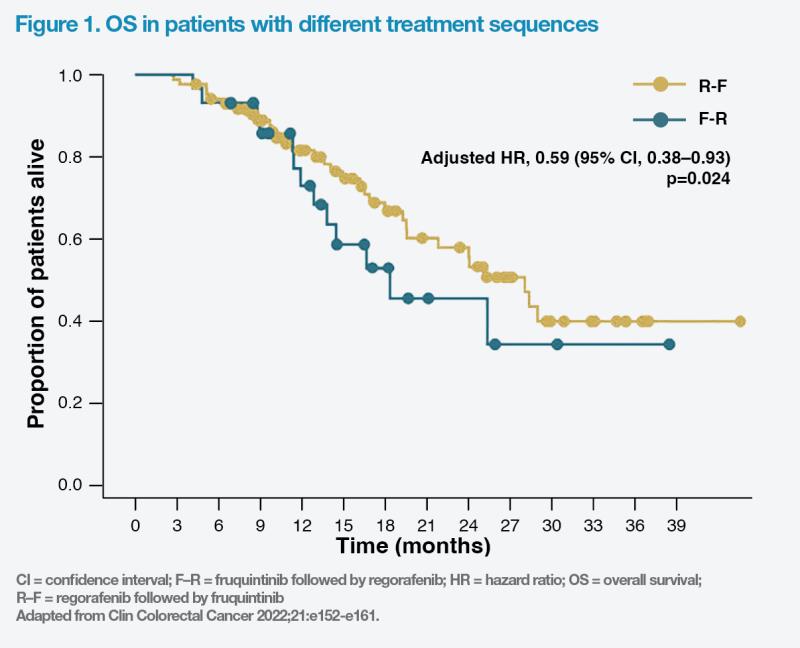
“In cases 2 and 3, we used regorafenib before fruquintinib, after earlier lines of systemic therapy, based on the patients’ relatively good ECOG PS and FRESCO-2 data, which included patients previously treated with regorafenib, and the proven real-world survival benefits associated with this sequence,” Sumou remarked. “With the regorafenib-fruquintinib sequence, both patients have survived for ≥48 months.”

Long-term regorafenib responders
Some patients with mCRC may experience long-term responses to regorafenib. A large-scale real-world retrospective cohort study (n=2,326) conducted in the US found that longterm responders (on treatment for ≥4 and ≥5 months) typically had favourable ECOG PS (0–1), less advanced disease at diagnosis, or prior therapy with bevacizumab. [J Clin Oncol 2024;doi:10.1200/JCO.2024.42.3_ suppl.48]
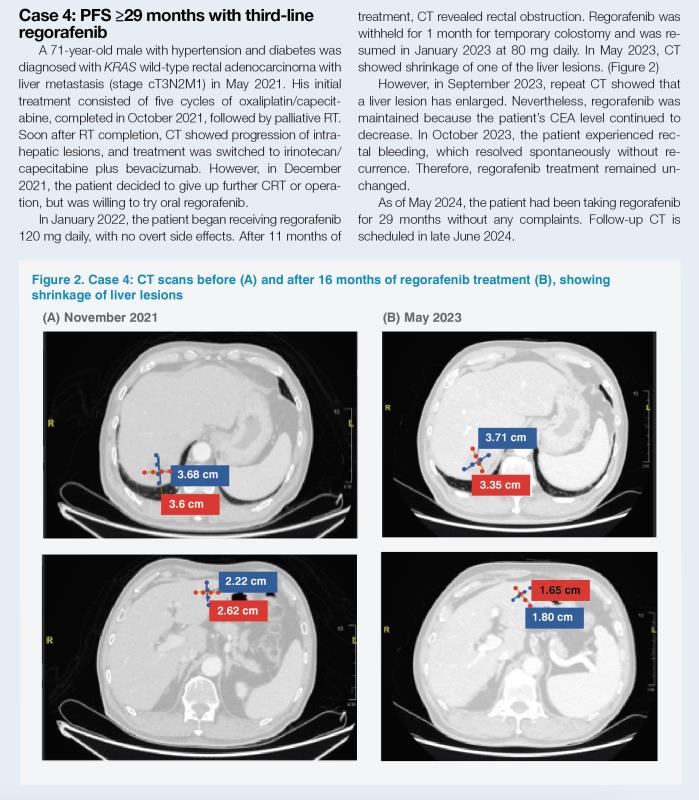
“The patients in cases 4 and 5 are long-term regorafenib responders from our practice. They both received regorafenib as third-line treatment, and their PFS was ≥29 months and ≥12 months, respectively, which is markedly longer than the mPFS reported in CORRECT and CONCUR. They both had prior bevacizumab and were good candidates for regorafenib initiation as they were fit and had good ECOG PS,” noted Sumou. (Table 1)

Safety of regorafenib and AE management
Despite its proven OS benefit in refractory mCRC, regorafenib’s toxicity profile, particularly HFSR, hepatotoxicity, and fatigue, which can lead to dose modifications or drug discontinuation, has limited its use in clinical practice. [Lancet Oncol 2015;16:619-629]
“Clinical trial data have shown that these AEs are most likely to occur during the first few cycles of treatment and tend to improve over time. As it is our aim to ensure that patients stay on treatment for as long as possible, our team prefers starting regorafenib at a dose lower than the standard dose [160 mg daily] and uptitrate gradually to minimize toxicities, which is based on the NCCN Guideline recommendation [Category 2A] and the ReDOS study,” pointed out Sumou. “This dose escalation strategy improves treatment compliance and works well in our patients, as illustrated in cases 4 and 5. Both patients were started on 120 mg of regorafenib, which was downtitrated to 80 mg due to AEs without ever titrating up to the standard dose, but they still had very long PFS.”
Optimized regorafenib dosing was shown to improve tolerability in the randomized, open-label, phase II ReDOS study. A significantly higher proportion of refractory mCRC patients received a regorafenib starting dose of 80 mg daily, which was subsequently uptitrated in weekly increments of 40 mg (n=54), and initiated cycle 3 vs patients who received the standard 160 mg daily dose from the start (n=62) (43 vs 26 percent; p=0.043). [Lancet Oncol 2019;20:1070-1082; NCCN Guidelines, Colon Cancer, version 2.2024; NCCN Guidelines, Rectal Cancer, version 2.2024]
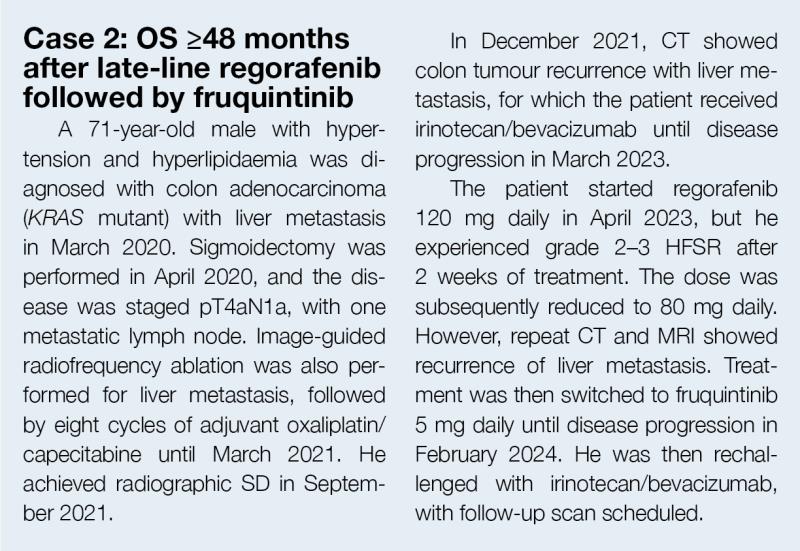
“Early and proactive prophylaxis and management of AEs are also important to ensure patients remain on treatment. Educating patients and their family members about the balance of risks and benefits and showing empathy can encourage patients to persist in treatment,” Sumou commented. “In case of intolerable side effects, we may reduce the dose or withhold treatment based on the dose modification algorithm outlined in the product label.” (Table 2)