OSHK Guideline updates for pharmacological management of postmenopausal osteoporosis





In Hong Kong, the number of hip fractures is forecast to increase over the next 3 decades. Patients who have experienced a major osteoporotic fracture (MOF) within the last 2 years are at very high risk of a second fracture. Dr Tai-Pang Ip, Chairperson of the Task Group for the Formulation of the 2024 Osteoporosis Society of Hong Kong (OSHK) Guideline for Clinical Management of Postmenopausal Osteoporosis, discussed pharmacological updates in the 2024 OSHK Guideline. A new treatment algorithm based on the levels of the individual’s fracture risk is formulated for management of postmenopausal osteoporosis.
Recent MOF increases imminent fracture risk
“The annual number of hip fracture cases in Hong Kong will continue to grow,” said Ip. In an Asian study, Hong Kong’s annual number of hip fractures was projected to nearly triple in the next 3 decades, from 9,590 cases in 2018 to 27,468 cases in 2050. This increase is projected to occur in both females (3.11-fold increase) and males (2.30- fold increase). [Osteoporos Sarcopenia 2018;4:16-21]
A retrospective analysis in Hong Kong reported that almost 50 percent of patients experienced a second fracture within 2 years of the initial MOF (ie, fractures of the hip, distal radius, or proximal humerus). [Arch Osteoporos 2019;14:104] “These results indicate that a maximal risk of imminent fracture occurs in the first 2 years after a MOF,” Ip pointed out.
2024 OSHK Guideline for osteoporosis management
“In 2013, OSHK developed a clinical guideline for osteoporosis management, which was the first locally published guideline to recommend osteoporosis treatment based on risk levels of osteoporotic fracture,” said Ip. The last 2013 OSHK Guideline recommended considering drug holidays upon risk reassessment after 3–5 years of bisphosphonate (BP) treatment. Similar recommendations regarding BP drug holidays were included in the 2016 American Society for Bone and Mineral Research Task Force report. [Hong Kong Med J 2013;19:1-40; J Bone Miner Res 2016;31:16-35]
Fracture risk stratification
“In the updated 2024 OSHK Guideline, patients are stratified into three groups. The very-high-risk group includes patients with multiple fractures, MOF within 2 years, T-score ≤-3.0 and fracture on antiresorptive therapy,” Ip explained. High-risk patients are those with a prior fracture >24 months ago, those aged ≥65 years with T-score ≤-2.5, and those assessed by the fracture risk assessment tool (FRAX) to have a 10-year probability of MOF of ≥20 percent or hip fracture of ≥3 percent. (Figure) [Hong Kong Med J 2024;30:S1-S44]
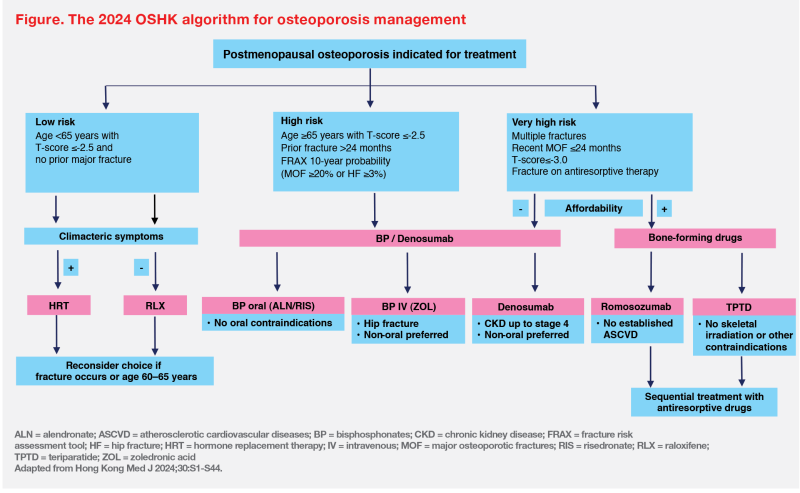
“The cut-off values for FRAX are, however, based on Caucasian data. Local cut-offs to indicate high risk remain to be defined,” he added.
“Similar fracture risk classification criteria have also been adopted by the 2020 International Osteoporosis Foundation [IOF] Osteoporosis Management Algorithm and the 2020 updated guidelines of American Association of Clinical Endocrinologists [AACE]/American College of Endocrinology [ACE],” pointed out Ip.
Timely use of bone-forming drugs for very-high-risk patients
“Regarding pharmacological management, the 2024 OSHK Guideline suggests timely use of bone-forming drugs for patients with very high fracture risk, if financially affordable,” said Ip. (Figure) [Hong Kong Med J 2024;30:S1-S44]
Bone-forming drugs stimulate bone formation, leading to a rapid and substantial increase in bone mineral density (BMD) over a short duration of treatment. These agents are shown to not only promote bone mass gain and improve bone microarchitecture, but also provide rapid and sustained reduction in fracture risk vs antiresorptive drugs. [J Clin Rheumatol Immunol 2022;22:10-19; J Bone Miner Res 2019;34:1597-1608; J Bone Miner Res 2003;18:1932-1941; N Engl J Med 2017;377:1417-1427; Lancet 2018;391:230-240]
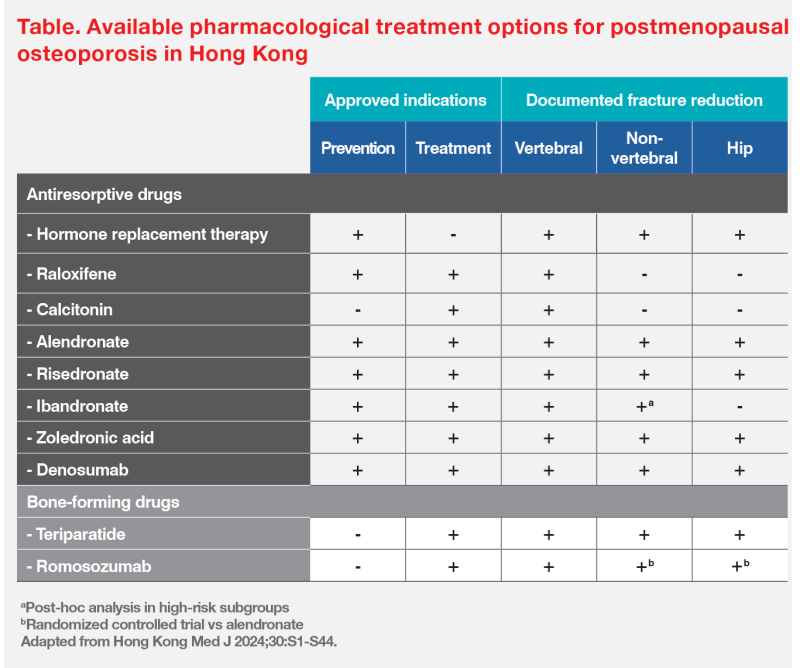
“Romosozumab and teriparatide are bone-forming drugs currently available [for treating postmenopausal osteoporosis] in Hong Kong,” said Ip. (Table) [Hong Kong Med J 2024;30:S1-S44]
Romosozumab: An anabolic agent with proven efficacy
Romosozumab reduced the risk of osteoporotic fracture vs alendronate (an antiresorptive agent) among postmenopausal women with osteoporosis and a fragility fracture in the phase III, head-to-head ARCH trial. In ARCH, patients were randomized to receive subcutaneous (SC) romosozumab (n=2,046) or oral alendronate (n=2,047) for 12 months, followed by up to 24 months of open-label alendronate in both groups. [N Engl J Med 2017;377:1417-1427]
The 24-month cumulative incidence of new vertebral fracture was 6.2 percent in the romosozumab-to-alendronate group vs 11.9 percent in patients given alendronate alone, translating to a 48 percent reduction in risk of new vertebral fracture in the romosozumab group (risk ratio, 0.52; 95 percent confidence interval [CI], 0.40–0.66; p<0.001). The romosozumab-to-alendronate strategy also demonstrated a 27 percent reduction in risk of clinical fracture vs alendronate alone (hazard ratio, 0.73; 95 percent CI, 0.61–0.88, p<0.001).
A post-hoc analysis of the phase III FRAME trial showed that romosozumab improved absolute total hip BMD T-score by 0.32 points at 12 months and 0.45 points at 24 months. When compared to the efficacy in BMD gain with denosumab in the FREEDOM trial and its extension study, the absolute increase in hip T-score achieved with 1 year of romosozumab followed by 1 year of denosumab treatment is similar to about 7 years of treatment with denosumab alone. These findings support the superior clinical benefits of rebuilding the skeletal foundation with romosozumab before antiresorptive therapy. [J Bone Miner Res 2018;33:1219-1226]
2024 OSHK Guideline recommendations on romosozumab
“The 2024 OSHK Guideline recommends 12-month treatment with romosozumab as one of the options for veryhigh- risk patients,” Ip noted. (Figure) “However, romosozumab is contraindicated in patients with a history of myocardial infarction or stroke. In the ARCH trial, the incidence of serious cardiovascular adverse events was 2.5 percent in the romosozumab group vs 1.9 percent in the alendronate group.” [Hong Kong Med J 2024;30:S1-S44; Evenity Hong Kong Prescribing Information; N Engl J Med 2017;377:1417-1427]
“To maintain BMD gains and reduce fracture risk, all patients should receive sequential denosumab or BPs after completing treatment with bone-forming agents,” he added. [Hong Kong Med J 2024;30:S1-S44]
Role of denosumab in high-risk patients
“In the 2024 OSHK Guideline, potent antiresorptive drugs such as denosumab or zoledronic acid are recommended for very-high-risk patients ineligible for [or unable to afford] bone-forming agents and high-risk patients,” Ip said. (Figure) [Hong Kong Med J 2024;30:S1-S44]
For patients treated with BPs, fracture risk should be re-evaluated after 5 years of oral therapy or 3 years of intravenous (IV) therapy. Osteoporosis therapy should be continued if patients remain at high fracture risk. Recent data showed that Asian patients are at a particular high risk of atypical femur fracture after 5 years of oral BP treatment. The 2024 OSHK Guideline recommends switching to denosumab if patients remain at high risk of fracture. [N Engl J Med 2020;383:743-53; Hong Kong Med J 2024;30:S1-S44]
In a pooled analysis of four phase III or IV randomized trials with 2,850 postmenopausal women who had received BPs, including oral and IV formulations, 12-month denosumab therapy significantly increased BMD from baseline vs BPs at tested skeletal sites (p<0.001 for all). The percentage increases in BMD from baseline with denosumab vs BPs were 2.0 percent (95 percent CI, 1.8–2.3) for lumbar spine, 1.3 percent (95 percent CI, 1.1–1.5) for total hip, 1.2 percent (95 percent CI, 1.0–1.4) for femoral neck and 0.6 percent (95 percent CI, 0.3– 1.0) for 1/3 radius. [Osteoporos Int 2020;31:181-191]
“If denosumab discontinuation is considered, an alternative antiresorptive drug must be given for prevention of bone loss. Drug holidays are inappropriate for denosumab-treated patients,” Ip emphasized. “Small-scale clinical studies showed that IV BPs after denosumab treatment may partially preserve bone mass, but the optimal BP regimen remains to be defined.”
“[However,] we encourage patients to continue treatment with denosumab if possible,” he added.
Summary
The 2024 OSHK Guideline for Clinical Management of Postmenopausal Osteoporosis defines fracture risk stratification and proposes a new management algorithm. Romosozumab is recommended as one of the bone-forming agents for patients with very high fracture risk. Denosumab is one of the first-line antiresorptive options in patients at high fracture risk and is an effective agent for switching after long-term treatment with BPs for patients who remain at high fracture risk.