
Presentation and management
A 75-year-old male presented with frequent urination in January 2023. His prostate-specific antigen (PSA) level was above normal at 54.2 ng/mL. Prostate biopsy yielded a Gleason score of 4 + 3. Prostate-specific membrane antigen (PSMA) PET-CT scan showed multiple bone metastases, with left supraclavicular lymph node (LN) and right external iliac LN uptake. The patient was diagnosed with high-volume metastatic castration-sensitive prostate cancer (mCSPC).
The patient's Eastern Cooperative Oncology Group (ECOG) performance status (PS) score was 2. He had a history of minor stroke and benign gastrointestinal stromal tumour. At diagnosis, he had multiple comorbidities requiring various comedications, including metoprolol tartrate 25 mg BID for hypertension, rosuvastatin 5 mg QD for hyperlipidaemia, and edoxaban 30 mg QD for atrial fibrillation. He had also undergone renal transplant due to end-stage kidney disease and was on cyclosporine A (150 mg OM and 125 mg PM), which replaced everolimus due to drug-drug interactions (DDIs).
In January 2023, the patient commenced androgen deprivation therapy (ADT) with degarelix Q1M, which was switched to leuprorelin Q3M in February 2023. A second-generation androgen receptor inhibitor (ARI), apalutamide (240 mg), was added in February 2023. One month after starting apalutamide, the patient’s urinary frequency improved along with a substantial decrease in PSA level from 54.2 ng/mL to 3.57 ng/mL.
In May 2023, the patient began to experience ankle oedema and pruritus, with grade 2 eczematous rash on limbs and trunk. He was treated with loratadine and antipruritic cream, and apalutamide’s daily dose was reduced to 180 mg. Although his ankle oedema improved, the rash persisted until July 2023. The dose of apalutamide was further reduced to 120 mg daily and he was given a steroid cream and another oral antihistamine (chlorphenamine), after which the rash gradually improved.
Notably, despite two dose reductions, the patient’s PSA level continued to drop consistently: from 3.57 ng/mL to 0.14 ng/mL after the first reduction and from 0.07 ng/mL to 0.03 ng/mL after the second.
As of October 2023, the patient remained on leuprorelin and apalutamide 120 mg daily, tolerating his treatment well.
Discussion
According to European Association of Urology’s guidelines, doublet therapy with ADT and an ARI is a recommended first-line option for mCSPC.1
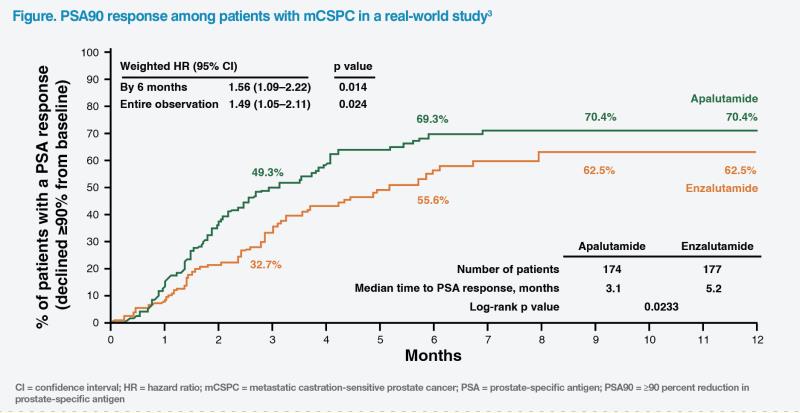
When choosing between available ARIs for our patient, we considered their ability to achieve ≥90 percent reduction in PSA (PSA90) within 3 months, which is associated with favourable prognosis in mCSPC.2 In a real-world study comprising 351 patients with mCSPC, apalutamide was associated with higher rates of PSA90 (70.4 percent vs 62.5 percent; hazard ratio, 1.49; 95 percent confidence interval, 1.05–2.11; p=0.024) and a shorter median time to PSA90 response (3.1 months vs 5.2 months) vs enzalutamide.3 (Figure)
In addition, compared with enzalutamide, apalutamide was associated with a longer time to development of castration resistance (CR), which is associated with poor prognosis in mCSPC.4 A retrospective, longitudinal cohort study of mCSPC patients (n=1,251) showed lower rates of CR progression at 12 months, 18 months and 24 months with apalutamide vs enzalutamide (12.4 percent, 23.5 percent and 26.2 percent vs 23.5 percent, 28.8 percent and 36.3 percent, respectively) and higher rates of CR-free survival (82.6 percent, 70.1 percent and 67.6 percent vs 71.5 percent, 62.6 percent and 53.9 percent, respectively).5
Apalutamide is generally well tolerated. The most common adverse events (AEs) of all grades reported in a randomized clinical trial were skin rash, fatigue, hypertension, and diarrhoea (vs placebo: 28 percent vs 9 percent, 20 percent vs 17 percent, 18 percent vs 16 percent, and 9 percent vs 6 percent, respectively), but the majority of these events were of grade 1–2.6 Despite reporting some AEs, patients in the ADT plus apalutamide arm of the phase III TITAN trial (n=1,052) maintained good health-related quality of life throughout the study (median follow-up, 44 months).7
In our practice, apalutamide-related skin reactions have not been encountered frequently and can generally be managed with an oral antihistamine and topical antipruritic and/or steroid cream. Haemoglobin level monitoring is advised for patients experiencing fatigue to eliminate possible ADT-related anaemia.8 Engaging in physical activity is also recommended to increase stamina. Blood pressure (BP) monitoring is advised for early detection of ARI-associated hypertension that may require treatment.9 Patients are instructed to measure their BP at home daily as “white-coat hypertension” might be encountered at the clinic. Mild diarrhoea is managed with antimotility medication. If mild AEs persist or in case of grade ≥3 AEs, apalutamide dose reduction or temporary suspension may be necessary.
ARI-related falls and resulting fractures are of concern for elderly patients, particularly those who are frail with ECOG PS score ≥2 or have comorbidities, such as visual impairment or history of stroke. Patients and their social support should be assessed individually, with caution exercised, when prescribing these agents.10
DDIs should also be considered when prescribing ARIs. Concomitant use of apalutamide with medications primarily metabolized by CYP3A4, CYP2C19 or CYP2C9 can lower their concentration. If apalutamide is co-administered with an anticoagulant metabolized by CYP2C9, such as warfarin, patients’ clotting profile should be closely monitored. Moreover, co-administration with a strong CYP2C8 or CYP3A4 inhibitor is predicted to increase exposure to apalutamide. Although initial dose adjustment is not necessary, apalutamide’s dose may be reduced based on tolerability.10
While ADT and ARI can be initiated at the same time, our patient was put on apalutamide 1 month after ADT initiation to allow a washout period for everolimus, a substrate of CYP3A4, to avoid potential DDIs.11 In spite of this delay, his PSA level declined substantialy within a month of starting apalutamide. Furthermore, his PSA level continued to drop even following two apalutamide dose reductions necessitated by rash.
Most importantly, our elderly patient with multiple comorbidities and concomitant medications remained asymptomatic with no sign of disease progression and maintained a good quality of life with ADT plus apalutamide treatment.