
Presentation and initial investigations
A 72-year-old male presented with symptomatic anaemia in July 2022. Initial peripheral blood examination revealed haemoglobin (Hb) level of 6 g/ dL, mean corpuscular volume (MCV) of 90 fL, white blood cell (WBC) count of 7.85 x 109/L, and platelet count of 93 x 109/L. His butyrate and folate levels, liver enzyme levels, and clotting profile were normal. His baseline erythropoietin (EPO) level was >500 U/L. There were no drug- or organ-related causes that could account for the anaemia. His past health was unremarkable apart from a history of chronic drinking.
Bone marrow aspirate showed dysplastic megakaryocytes, dyserythropoiesis, and 15 percent ring sideroblasts (RS), which led to the diagnosis of myelodysplastic syndrome (MDS). The myeloid lineage was normal. His diagnosis was further confirmed with cytogenetic analysis. Molecular testing with next-generation sequencing (NGS) detected the presence of SF3B1 (hotspot K700E) mutation with a variant allele frequency (VAF) of 40 percent. His disease was categorized as low-risk (LR)-MDS based on the Revised International Prognostic Scoring System (IPSS-R).
Treatment
In August 2022, the patient was started on weekly injections of darbepoetin 120 μg, an erythropoiesis-stimulating agent (ESA), but failed to show any response. He remained transfusion-dependent, receiving two units of packed red blood cells (RBCs) every 4 weeks. After 6 months, it was evident that ESA treatment had failed. Additionally, his serum ferritin level was approximately 5,000 ng/mL, indicative of iron overload.
On 2 March 2023, luspatercept treatment was initiated at 1 mg/kg. His Hb levels remained at ≥7 g/dL during the following weeks, which obviated the need for further blood transfusions after the first dose of luspatercept. Over the following months, luspatercept dose was gradually increased to the maximum dose of 1.75 mg/kg in order to achieve and maintain a target Hb level of >9–9.5 g/dL for maximum symptom relief. This resulted in steady increases in Hb level and platelet count. (Figure)
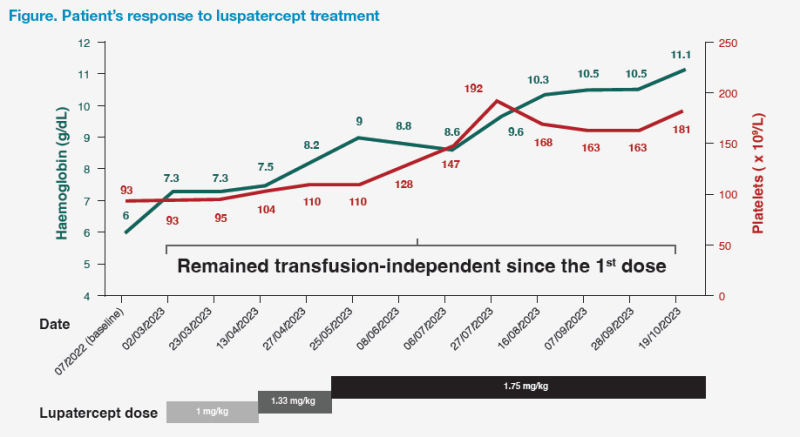
The patient experienced improvements in quality of life (QoL), as evidenced by improved anaemic symptoms, including malaise, fatigue, heart palpitations, and exercise intolerance. His energy levels significantly improved after his Hb level increased to >9 g/dL, allowing him to ambulate freely in the community. Furthermore, his serum ferritin level continued to decline to 900 ng/mL by 9 November 2023 without the need for iron chelation therapy.
As of November 2023, the patient had been on luspatercept for >8 months and remained transfusion-independent since the first dose of luspatercept. He tolerated the drug well, without experiencing any adverse events (AEs) and no longer had iron overload.
Discussion
Blood transfusions to treat MDS-related anaemia pose a significant burden on patients, caregivers and the healthcare system. Patients face risks of transfusion reactions (including haemolytic and febrile nonhaemolytic reactions), fluid and iron overload, and infections, and have reduced QoL from frequent hospital admissions.1 Our patient developed iron overload after 7 months of blood transfusions. Iron overload in MDS patients is caused not solely by blood transfusions, but also by increased iron absorption in the gut because of suppression of hepcidin.2
Luspatercept, an erythroid maturation agent, downregulates the transforming growth factor–beta (TGF-β) signalling pathway, resulting in improved erythroid maturation.3,4 Mutations in SF3B1 strongly correlate with the presence of bone marrow RS.5
Before considering treatment with luspatercept, all patients who have failed first-line treatment should be assessed for and excluded if there is evidence of disease progression to higher-risk MDS or if there are other causes of anaemia, such as bleeding or deficiencies of iron, butyrate or folate.
Therapeutic goals for LR-MDS include increase in Hb levels, achievement of transfusion independence and improvement/maintenance of QoL (eg, iron overload, physical, role and social functioning, fatigue, and dyspnoea).6,7 At our centre, we define transfusion independence as when patients’ Hb levels reach >7 g/dL for asymptomatic patients or Hb >9 g/dL for symptomatic patients.
Our patient had a high transfusion burden requiring two units of RBCs every 4 weeks. Remarkably, after initiating luspatercept, he achieved immediate transfusion independence and maintained Hb levels at ≥7 g/dL. In our centre, most patients who begin treatment with luspatercept achieve early transfusion independence or an early reduction in transfusion burden. A reduction in transfusion requirement is typically seen after two doses, followed by achieving transfusion independence and a rise in Hb levels after another four doses from previous experience. Moreover, a substantial increase in Hb level can be expected after dose escalations. Hb levels of >9–9.5 g/dL are optimal for maximum symptom relief. Additionally, our patient’s platelet counts increased during treatment with luspatercept, which was consistent with the MEDALIST trial.8 (Figure)
Luspatercept dose can be escalated every 6 weeks (two consecutive administrations) to a maximum of 1.75 mg/kg every 3 months for patients who are not transfusion-free, provided there are no persistent treatment-related grade ≥3 AEs, such as persistent or exacerbations of pre-existing hypertension, which should be treated.3 In the phase III, randomized MEDALIST study, 77.1 percent of all patients receiving luspatercept had their dose increased at least once.9
If there is loss of response to luspatercept treatment (ie, loss of transfusion independence), it is important to rule out other causes, including disease progression and compliance issues. Subsequently, dose escalation should be considered. Once the ceiling dose of 1.75 mg/kg is reached, it may be maintained for 9 weeks before stopping treatment if patients do not experience a reduction in transfusion burden.3
In the clinical trial setting, the majority of adverse reactions with luspatercept were of low grade and seldom led to treatment discontinuation.6
Luspatercept is indicated for patients with transfusion-dependent anaemia due to very low- to intermediate-risk MDS with RS who had an unsatisfactory response to or are ineligible for EPO-based therapy (eg, ESA).3 Patients with bone marrow blast <5 percent or SF3B1 mutation without other high-risk mutations, such as our patient, are most likely to benefit from second-line treatment with luspatercept.
Second-line treatment with luspatercept can reduce the need for RBC transfusions, which could lead to maintenance of QoL, and ultimately result in longer-term advantages for patients, caregivers, and the healthcare system.6,7