SGLT2i as 1L therapy for CKD: How to optimize real-world prescription?









Kidney Disease: Improving Global Outcomes (KDIGO) 2024 clinical practice guideline recommends sodium-glucose cotransporter 2 inhibitors (SGLT2i) as first-line (1L) therapy for most patients with chronic kidney disease (CKD), with or without diabetes. Despite the established cardiorenal benefits of SGLT2i, prescription remains suboptimal in real-world CKD populations. At Advances in Medicine (AIM) 2024, Professor Jennifer Green of Duke University Medical Center in Durham, North Carolina, US, discussed empagliflozin’s cardiorenal benefits in broad CKD populations and the importance of early initiation of SGLT2i therapy in delaying CKD progression, while Dr Jack Ng of the Chinese University of Hong Kong presented strategies for optimizing SGLT2i prescription in CKD.
SGLT2i: 2024 KDIGO-recommended 1L therapy for CKD
KDIGO’s 2024 CKD guideline recommends SGLT2i as 1L therapy for most patients with CKD, with or without type 2 diabetes (T2D). [Kidney Int 2024;105(4S):S117-S314]
“SGLT2i therapy is now recommended in adults with CKD with estimated glomerular filtration rate [eGFR] ≥20 mL/min/1.73 m2 and urine albumin-to-creatinine ratio [UACR] ≥200 mg/g or ≥20 mg/mmol, or those with heart failure irrespective of level of albuminuria [1A recommendation],” said Ng. “This is an expansion of KDIGO’s 2022 recommendation of SGLT2i as 1L therapy in patients with CKD and T2D.” [Kidney Int 2022;102:S1-S127]
“[Once initiated,] SGLT2i therapy is to be continued until dialysis or transplant,” noted Green.
The latest KDIGO recommendations are supported by randomized controlled trials demonstrating SGLT2i’s cardiorenal benefits in CKD patients, the most recent of which is the EMPA-KIDNEY trial of empagliflozin vs placebo.
Empagliflozin’s cardiorenal benefits in broad CKD population
“In EMPA-KIDNEY, empagliflozin safely reduced the risk of kidney disease progression or cardiovascular [CV] death by 28 percent vs placebo in a broad population of patients with CKD with various causes, including large numbers of patients with poor kidney function and mild or moderate albuminuria,” said Green. [N Engl J Med 2023;388:117-127]
The trial included CKD patients with eGFR 20–<45 mL/min/1.73 m2, or eGFR 45–<90 mL/min/1.73 m2 and UACR ≥200 mg/g, who were taking a clinically appropriate dose of a single-agent renin-angiotensin system inhibitor.
A total of 6,609 patients (mean age, 63.8 years; female, 33.2 percent) were randomized to receive empagliflozin 10 mg QD (n=3,304) or placebo (n=3,305). At baseline, the patients’ mean eGFR was 37.3 mL/ min/1.73 m2, and 34.5 percent had eGFR <30 mL/min/1.73 m2. Median UACR was 329 mg/g, and 48.3 percent had UACR <30 mg/g (A1 albuminuria) or 30–300 mg/g (A2 albuminuria). Fifty-four percent of patients had no history of diabetes, while 69 percent had nondiabetic causes of CKD.
Reduced risk of CKD progression or CV death
After a median follow-up of 2.0 years, empagliflozin demonstrated a 28 percent relative risk reduction (RRR) in the primary composite outcome of CKD progression (ie, end-stage kidney disease [ESKD], a sustained decrease in eGFR to <10 mL/ min/1.73 m2, a sustained decrease in eGFR of ≥40 percent from baseline, or death from renal causes) or death from CV causes vs placebo (hazard ratio [HR], 0.72; 95 percent confidence interval [CI], 0.64– 0.82; p<0.001). (Figure 1) [N Engl J Med 2023;388:117-127]
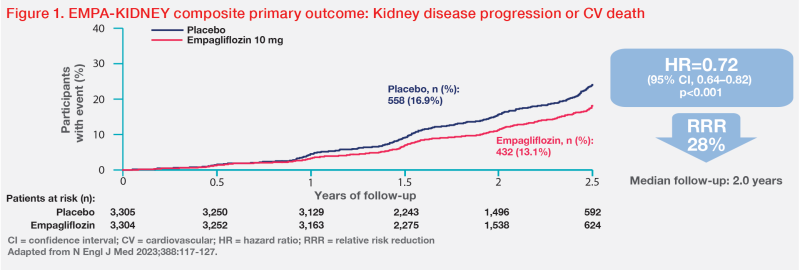
“The benefit was fairly consistent among key subgroups of interest, including patients with or without diabetes at baseline and those with different eGFRs,” said Green.
Similar RRRs in kidney disease progression (HR, 0.71; 95 percent CI, 0.62–0.81) and ESKD or CV death (HR, 0.73; 95 percent CI, 0.59–0.89) were observed with empagliflozin vs placebo.
Slower eGFR decline across eGFR and UACR subgroups
Annual rate of change in eGFR (a surrogate for kidney disease progression) was -1.37 vs -2.75 mL/min/1.73 m2 in the empagliflozin vs placebo group between 2 months and final follow-up (ie, long-term eGFR slope), with a between-group difference of 1.37 mL/min/1.73 m2/year favouring empagliflozin. [Am J Kidney Dis 2020;75:84-104; N Engl J Med 2023;388:117-127]
“Empagliflozin’s effect on long-term eGFR slope was quite consistent irrespective of baseline diabetes status,” said Green.
The findings also highlight the importance of early SGLT2i therapy in preserving kidney function. “Empagliflozin’s benefit in long-term eGFR slope was greater in patients with higher eGFR,” Green pointed out. “Although greater benefit was seen in patients with more severe albuminuria, in patients with A1 albuminuria, in whom there were very few primary outcome events [n=84/1,328 in total], empagliflozin almost entirely mitigated eGFR decline over time, to a negligible rate of -0.11 mL/min/1.73 m2/year.” (Figure 2)
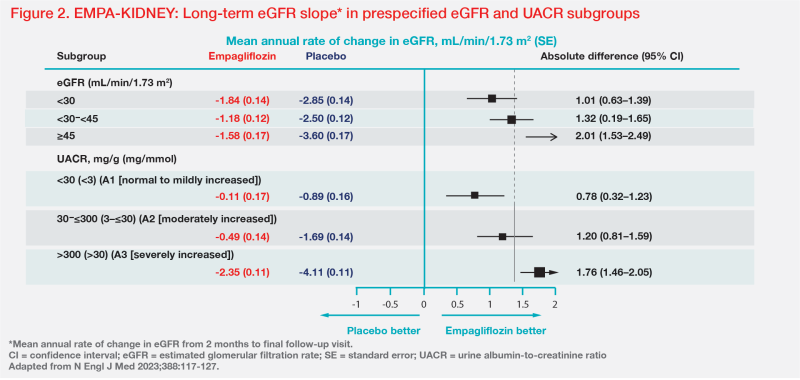
Real world: Why are SGLT2i underprescribed in CKD?
SGLT2i remain underprescribed in real-world CKD populations despite their proven cardiorenal benefits and previous guideline recommendations.
In a cross-sectional study involving 516,491 patients with CKD registered with UK primary care practices on 31 December 2022, 26.8 percent had a guideline-directed indication for SGLT2i, but only 17.0 percent of patients with an indication actually received treatment. “Paradoxically, those with eGFR <60 mL/min/1.73 m2 or microalbuminuria [UACR, 3–30 mg/mmol], who are at highest risk of deterioration, were less likely to be prescribed SGLT2i,” said Ng. [EClinicalMedicine 2024;68:102426]
UACR testing underutilized
Among patients who were reported not to fulfil guideline criteria for SGLT2i therapy in the above UK primary care study, approximately one-third lacked UACR assessment. [EClinicalMedicine 2024;68:102426]
“UACR testing is often underutilized. In a study involving 192,108 patients with hypertension or diabetes in the US, only 35.2 percent of the projected population with albuminuria had undergone UACR testing,” said Ng. “UACR assessment was associated with significantly higher odds of SGLT2i use [odds ratio, 8.22; 95 percent CI, 7.56–8.94; p<0.001].” [JAMA Netw Open 2023;6:e2326230]
Knowledge gap, time constraints
In Hong Kong, semi-structured interviews with 17 primary care physicians (practice duration >10 years, 58.8 percent) in the public healthcare sector, conducted between January and May 2021, revealed a knowledge gap that affects SGLT2i prescription in patients with T2D and established CV disease or CKD. [BMC Prim Care 2022;23:317]
“Most interviewees were aware of the cardiorenal benefits of SGLT2i, but were reluctant to prescribe SGLT2i because some of them were not fully aware that the benefits are independent of glycaemic efficacy,” said Ng. “Some had concerns about SGLT2i use in the elderly. However, data from the EMPA-REG OUTCOME trial showed that empagliflozin’s benefit is preserved in elderly patients, with no new safety signals identified.” [BMC Prim Care 2022;23:317; Age Ageing 2019;48:859-866]
Among nephrologists (n=161) who responded to a global online survey, time constraints and lack of competence were common barriers to SGLT2i prescription. Only 55 percent prescribed SGLT2i to >50 percent of their CKD patients with T2D and proteinuria. [Kidney Int Rep 2023;8:1669-1671]
How to optimize SGLT2i prescription in CKD?
“Enhancing albuminuria screening and fostering interdisciplinary care are some of the strategies that may optimize SGLT2i prescription,” commented Ng.
Enhancing albuminuria screening
Bundling eGFR and UACR into a kidney profile panel can simplify the test ordering process for primary care physicians, thus facilitating early CKD diagnosis and risk stratification. [https://www.kidney.org/ news/national-kidney-foundation-american-society-clinical-pathology-leading-laboratories-and]
“I put urine bottles on the desk as a reminder to order UACR, and ask patients to take urine bottles before collecting appointment slips and medications,” said Ng.
“UACR is recommended over urine protein-to-creatinine ratio [UPCR] for albuminuria assessment. When UACR is unavailable, an equation that converts UPCR to UACR may be used,” he noted. “The conversion equation is accessible online, free of charge, and has demonstrated moderate sensitivity [87 percent] and high specificity [98 percent] in detecting A3 albuminuria [UACR >300 mg/g].” [Ann Intern Med 2020;173:426-435]
Units matter: mg/mmol vs mg/g
“Standardization is lacking in measurement units and description terms used by laboratories when reporting UACR results. This may lead to misinterpretation of results that jeopardizes SGLT2i prescription,” said Ng.
Confusion of measurement units is common. In Ng’s survey of 13 non-nephrologists and non-endocrinologists, only 38.5 percent correctly identified 30 mg/g (not 30 mg/mmol) as cut-off for A2 microalbuminuria. [Ng J, AIM 2024]
“There is an approximately 10-times difference between mg/g and mg/mmol. The KDIGO threshold for SGLT2i treatment in CKD is UACR ≥200 mg/g or ≥20 mg/mmol,” he pointed out. [Kidney Int 2024;105(4S):S117-S314] “Reporting UACR results in both units helps minimize misinterpretation and confusion.”
Fostering interdisciplinary care
For primary care physicians who are at the forefront of CKD screening, KDIGO has developed top 10 takeaways on CKD evaluation and management based on its 2024 guidelines. [https://kdigo.org/guidelines/ ckd-evaluation-and-management/]
“Specialists should reinforce interdisciplinary care for patients with comorbidities,” suggested Ng. “An interdisciplinary and multidisciplinary cardiac-renal-endocrine clinic provides one-stop, coordinated and cohesive care for patients with comorbid cardio-renal-metabolic conditions. This approach has been shown to improve uptake of evidence-based medications, including SGLT2i, as well as patients’ blood pressure, HbA1c and LDL-cholesterol levels.” [Can J Kidney Health Dis 2022;doi:10.1177/20543 581221081207]
Summary
SGLT2i are recommended by KDIGO’s 2024 guideline as 1L therapy for CKD. Empagliflozin’s cardiorenal benefits demonstrated in the EMPA-KIDNEY trial support its use in broad CKD populations. In real-world settings, enhanced albuminuria screening and fostering interdisciplinary care may facilitate increased prescription of SGLT2i in CKD patients.