
At the Society of Gynecologic Oncology (SGO) 2024 Annual Meeting on Women’s Cancer held in San Diego, US, researchers presented updated results from the two-part, phase III ENGOT-EN6-NSGO/GOG-3031/RUBY trial, demonstrating statistically significant and clinically meaningful improvements in overall survival (OS) and progression-free survival (PFS) associated with dostarlimab-based combinations in patients with primary advanced or recurrent endometrial cancer (EC).
Background
For over two decades, chemotherapy has been the standard of care for first-line treatment of primary advanced or recurrent EC. However, long-term outcomes have remained dismal with median OS <3 years. [J Adv Pract Oncol 2022;13:45-59; J Clin Oncol 2020;38:3841-3850; Gynecol Oncol 2020;157:312-322]
Combining chemotherapy with immunotherapy may synergistically improve treatment outcomes in these patients. Dostarlimab is a humanized monoclonal antibody that binds to the programmed cell death-1 (PD-1) receptor and blocks its interaction with the PD-1 ligands PD-L1 and PD-L2. [Eur J Cancer 2016;69(Suppl 1):S102] In the two-part, double-blind, multicentre RUBY trial, the safety and efficacy of dostarlimab in combination with chemotherapy were evaluated in patients with primary advanced or recurrent EC.
Previously reported initial interim analysis of RUBY Part 1 showed significant improvements in PFS in the mismatch repair–deficient/microsatellite instability–high (dMMR/MSI-H) and overall populations, with an early OS trend associated with dostarlimab plus chemotherapy vs chemotherapy alone. [N Engl J Med 2023;388:2145-2158] These findings led to regulatory approval of dostarlimab plus chemotherapy for patients with dMMR/MSI-H primary advanced or recurrent EC in several countries. [https://www.ema.europa.eu/en/medicines/human/EPAR/jemperli; https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-dostarlimab-gxly-chemotherapy- endometrial-cancer]
In Hong Kong, dostarlimab is currently indicated in combination with carboplatin and paclitaxel (CP) for treatment of adult patients with dMMR/MSI-H primary advanced or recurrent EC who are candidates for systemic therapy, and as monotherapy for treatment of adult patients with dMMR/MSI-H recurrent or advanced EC that has progressed on or following prior treatment with a platinum-containing regimen. [Jemperli Hong Kong Prescribing Information, 2023]
RUBY Part 1: Second interim analysis
In Part 1 of RUBY, 494 patients with primary advanced or recurrent EC were randomized 1:1 to receive dostarlimab plus CP followed by maintenance dostarlimab (n=245), or placebo plus CP followed by placebo (n=249). [N Engl J Med 2023;388:2145-2158]
Eligible patients had stage III/IV disease or first recurrence of EC. Patients could be naive to systemic anticancer therapy or have disease recurrence or progression within 6 months after completion of systemic anticancer treatment. At the first interim analysis, the dual primary endpoints were investigator-assessed PFS in both the overall and dMMR/MSI-H populations and OS in the overall study population.
OS was the primary endpoint in the second interim analysis. Prespecified OS analyses were performed in the overall population as well as dMMR/MSI-H and mismatch repair–proficient/microsatellite stable (MMRp/MSS) populations. PFS2 was a secondary endpoint. [Powell MA, et al, SGO 2024]
At baseline, 87 percent of patients had measurable disease and 76 percent had MMRp/MSS status.
OS benefit
After a median follow-up of 37.2 months, a statistically significant and clinically meaningful improvement in OS was observed for dostarlimab plus CP vs placebo plus CP in the overall population.“There was a significant 31 percent improvement in OS [hazard ratio (HR), 0.69; 95 percent confidence interval (CI), 0.54–0.89; p=0.002] and a corresponding 16.4-month increase in median survival for patients in the dostarlimab arm – despite 38.2 percent of patients in the placebo arm receiving subsequent immunotherapy vs 17.1 percent in the dostarlimab arm,” noted principal investigator, Dr Matthew Powell of the Washington University School of Medicine, St Louis, US. (Figure 1) [Powell MA, et al, SGO 2024]
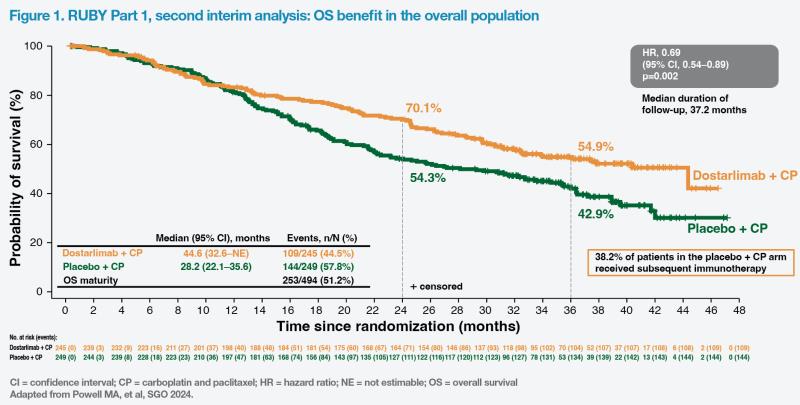
In the dMMR/MSI-H population, at 39.8 percent OS data maturity, median OS was not estimable in the dostarlimab plus chemotherapy arm and 31.4 months in the placebo plus chemotherapy arm. “There was an unprecedented 68 percent improvement in survival for dMMR/MSI-H patients who received dostarlimab plus chemotherapy [HR, 0.32; 95 percent CI, 0.17–0.63; p=0.0002], even though 41.5 percent of patients in the placebo arm went on to receive subsequent immunotherapy vs 15.1 percent in the dostarlimab arm,” commented Powell.
The investigators also reported a clinically meaningful 21 percent reduction in risk of death (HR, 0.79; 95 percent CI, 0.60–1.04; p=0.0493) and a 7-month improvement in median OS in MMRp/MSS patients who received dostarlimab plus chemotherapy vs placebo plus chemotherapy.
PFS2 benefit
Consistent with the OS results, there was a clinically meaningful difference in PFS2 favouring dostarlimab in the overall (median, 32.3 months vs 18.4 months; HR, 0.66; 95 percent CI, 0.52–0.84) and MMRp/MSS (median, 24.6 months vs 15.9 months; HR, 0.74; 95 percent CI, 0.57–0.97) populations. PFS2 difference between the two arms was especially substantial in the dMMR/MSI-H population (median, not estimable vs 21.6 months; HR, 0.33; 95 percent CI, 0.18– 0.63). [Powell MA, et al, SGO 2024]
Safety results
“Safety was maintained and no new deaths related to therapy were noted during the year following the initial analysis,” said Powell. “Most treatment-emergent adverse events [TEAEs] were of grade 1 or 2, except for anaemia, which was often grade 3, but manageable.” [Powell MA, et al, SGO 2024]
“These data confirm dostarlimab plus CP as a new standard of care for patients with primary advanced or recurrent EC, regardless of their mismatch repair status,” concluded Powell.
RUBY Part 2: Preliminary interim analysis
Following the remarkable survival benefits demonstrated at the initial interim analysis of Part 1 of the RUBY trial, Part 2 was designed to test if adding niraparib, a poly (ADP-ribose) polymerase inhibitor (PARPi), to dostarlimab for maintenance therapy may further improve outcomes, including in patients with MMRp/MSS disease, a population with high unmet need. [Mirza MR, et al, SGO 2024]
In Part 2, 291 patients with primary advanced or recurrent EC were randomized 2:1 to receive dostarlimab plus CP followed by maintenance dostarlimab plus niraparib (treatment arm; n=192), or placebo plus CP followed by placebo (control arm; n=99). The primary endpoint was investigator-assessed PFS, in both the overall and MMRp/MSS populations.
Baseline patient characteristics were well-balanced. A quarter of the study population had dMMR/MSI-H disease status and approximately half had locally advanced disease. At data cut-off (median follow-up of 19 months), 44 patients in the experimental arm and 20 patients in the placebo arm remained on study. The trial is ongoing for OS follow-up.
PFS benefit
“For PFS in the overall population, there was a statistically and clinically significant risk reduction of 40 percent [HR, 0.60; 95 percent CI, 0.43–0.82; p=0.0007], with an improvement in median PFS of 6.2 months [in the treatment arm],” reported co-principal investigator, Dr Mansoor Raza Mirza of the Copenhagen University Hospital, Copenhagen, Denmark. (Figure 2) [Mirza MR, et al, SGO 2024]
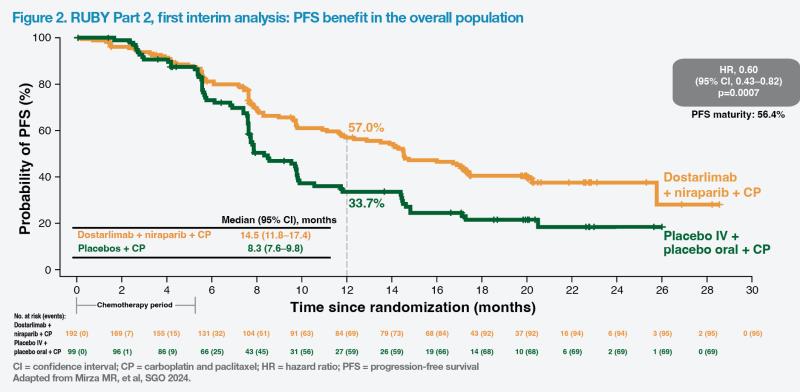
Similar statistically and clinically significant improvements in PFS were reported in the MMRp/MSS population. The risk of disease progression or death was reduced by 37 percent (HR, 0.63; 95 percent CI, 0.44–0.91; p=0.0060) and median PFS was 6.0 months longer in the treatment vs control arm (14.3 months vs 8.3 months).
The results also showed a clinically relevant benefit in the prespecified exploratory endpoint of PFS among a small number of patients with dMMR/MSI-H disease (n=75) treated with dostarlimab plus chemotherapy and maintained on dostarlimab and niraparib vs the control arm (HR, 0.48; 95 percent CI, 0.24–0.96; nominal p=0.0174).
Improvements with dostarlimab plus niraparib maintenance were also evident in other exploratory PFS analyses performed in various molecular subgroups, including patients with TP53 mutation, no specific molecular profile (NSMP), and PD-L1–positive status (combined positive score [CPS] ≥1). However, the investigators warned that these results should be interpreted with caution as the study was not powered to detect a treatment difference in any of these subgroups, and there were small numbers and low data maturity in some subgroups.
Safety results
Overall, TEAEs occurred in 99.5 percent and 100 percent of patients in the treatment and control arms, respectively. Grade ≥3 TEAEs were reported in 84.8 percent and 49.0 percent of patients, respectively. “With the exception of anaemia, the rates of grade ≥3 TEAEs were quite low,” highlighted Mirza. “The safety profile observed was generally consistent with the known safety profiles of the individual agents.”
“RUBY Part 2 met its primary endpoint, showing significant and clinically meaningful improvement in PFS for dostarlimab plus chemotherapy followed by dostarlimab plus niraparib maintenance in the overall and MMRp/MSS populations. These outcomes demonstrate a potential role for PARPi maintenance in patients receiving dostarlimab plus chemotherapy, particularly for those with MMRp/MSS disease,” summarized Mirza.