Eosinophilic granulomatosis with polyangiitis (EGPA) is a rare eosinophilic autoimmune disease that affects multiple organs. The lack of disease awareness and heterogeneous nature of clinical manifestations have made diagnosis and management of EGPA challenging. At Advances in Medicine (AIM) 2024, Professor Hiromichi Tamaki, a rheumatologist from St. Luke’s International Hospital in Japan, discussed how EGPA is diagnosed, presented real-world evidence on mepolizumab (an anti–interleukin [IL]-5 therapy) in EGPA and illustrated mepolizumab’s clinical benefits through several patient cases.
Eosinophils in EGPA pathogenesis
EGPA (formerly known as Churg- Strauss syndrome) is a rare, multiorgan, inflammatory, eosinophil-rich disease. [Autoimmun Rev 2023;22:103219]
“Eosinophils, the predominant cell type involved in EGPA inflammation, are known to play a role in regulation of homeostasis,” said Tamaki. Nonapoptotic cell death of activated eosinophils (ie, via cytolysis and subsequent release of cytotoxic proteins) can lead to eosinophilic inflammation and hypereosinophilic diseases, such as EGPA. [Front Immunol 2023;14:1170035; Eur Respir Rev 2022;31:210150; Mayo Clin Proc 2021;96:2694-2707; Allergol Int 2021;70:19-29]
Diagnosis of EGPA
Because of the rarity of the disease, diagnostic criteria for EGPA are lacking. Classically, EGPA progresses through three phases: prodromal phase, characterized by asthma and allergic rhinitis; eosinophilic phase, during which eosinophilia and tissue infiltration occur; and vasculitic phase, characterized by clinical manifestations due to small-to-medium vessel vasculitis and extravascular granulomatous inflammation. [Nat Rev Rheumatol 2023;19:378-393] “However, these phases often overlap and do not necessarily develop sequentially,” pointed out Tamaki.
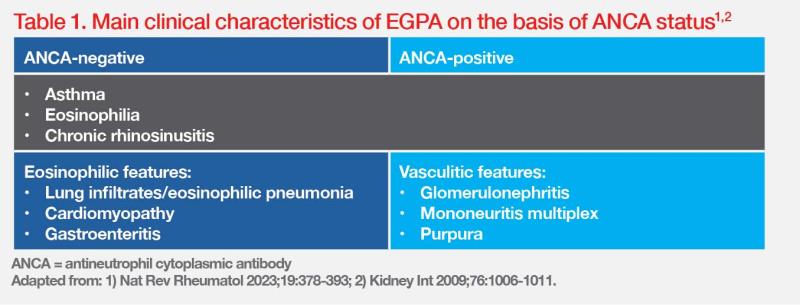
“[As demonstrated in cases 1–3,] when patients present with classic EGPA complications along with pre-existing asthma, chronic rhinosinusitis, and eosinophilia [>10 percent or >1,000 cells/ μL], clinical suspicion of EGPA should be raised,” highlighted Tamaki. (Table 1) [Nat Rev Rheumatol 2023;19:378-393; Biomedicines 2023;11:776]
“Clinical manifestations of EGPA often overlap with other vasculitic or eosinophilic disorders, such as hypereosinophilic syndrome [HES], eosinophilic asthma, and eosinophilic pneumonia,” added Tamaki. “Diagnostic evaluation should be performed with a multidisciplinary approach to rule out other eosinophilic and vasculitic disorders.”
“Antineutrophil cytoplasmic antibody [ANCA] positivity can support a diagnosis of EGPA. ANCA status should therefore be determined in all suspected cases,” said Tamaki. However, it should be noted that ANCA is not detectable in approximately 60 percent of EGPA cases, as shown in case 1.
Increasing evidence suggests clinical distinctions between ANCA-positive and ANCA-negative subsets of EGPA, with features of ANCA-associated vasculitis occurring more frequently in ANCA-positive patients, whereas eosinophilic features are more frequent in ANCA-negative patients. (Table 1) “Nevertheless, the clinical value of ANCA positivity should not be overestimated because of the substantial overlap between the ANCA-positive and ANCA-negative phenotypes,” Tamaki added. [Nat Rev Rheumatol 2023;19:378-393; Kidney Int 2009;76:1006-1011]
“Biopsy is recommended, when feasible, for histological evidence of vasculitis,” Tamaki highlighted.

Real-world experience with mepolizumab in EGPA
“Since IL-5 exerts a central pathogenic role in maturation, recruitment, migration and activation of eosinophils, anti–IL-5 therapy is a promising therapeutic option for EGPA treatment,” highlighted Tamaki.
Mepolizumab is a humanized monoclonal antibody targeting IL-5, which is currently indicated as an add-on treatment for adults with relapsing-remitting or refractory EGPA. [Nucala Hong Kong Prescribing Information]
Clinical experience at St. Luke’s International Hospital
“The three cases from our hospital all demonstrated mepolizumab’s OCS-sparing effect,” noted Tamaki.
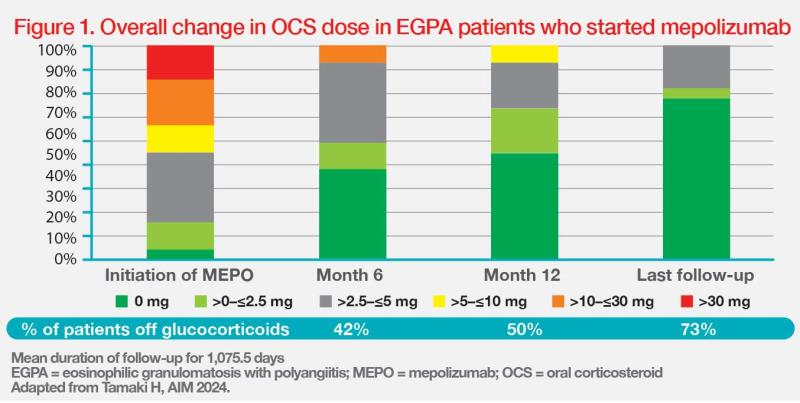
“To date, we have treated 26 patients with EGPA [median age, 61 years] with a median disease duration of 252 days at mepolizumab initiation. At baseline, most of them [96 percent] were using OCS, with a median dose of oral prednisolone of 8.5 mg/day,” noted Tamaki. “More importantly, mepolizumab treatment resulted in substantial OCS dose reduction. The patients’ median daily dose of prednisolone was decreased to 2 mg and 0 mg at months 6 and 12, respectively, and remained at 0 mg after a median follow-up of approximately 3 years.”
“Notably, many patients had their OCS downtitrated since mepolizumab was started, with half of the patients discontinuing OCS at 1 year and 73 percent of patients discontinuing OCS at 3 years,” highlighted Tamaki. (Figure 1)
MARS: Real-world study in Japan
The ongoing MARS study in Japan (n=116) recruited patients with EGPA who have completed a prior 96-week mepolizumab postmarketing surveillance study to receive mepolizumab for another 96 weeks. The 48-week interim results showed a very low rate of EGPA relapse (5 percent) with mepolizumab. Moreover, the median OCS daily dose decreased from 6.9 mg pre-mepolizumab to 3.0 mg at baseline and 2.0 mg at weeks 45–48, and the proportion of patients receiving no OCS increased from 8 percent to 32 percent and 38 percent, respectively. The proportion of patients experiencing clinical symptoms also decreased from 94 percent to 73 percent and 67 percent, respectively. [Mod Rheumatol 2023:road109]
“Safety results were consistent with the previously established safety profile of mepolizumab. Remarkably, mepolizumab was very well tolerated with no drug-related adverse events,” stressed Tamaki.
Potential benefit in preventing vasculitis
“Autoantibodies are known to develop years prior to clinical manifestation in certain autoimmune diseases, such as rheumatoid arthritis and lupus. However, we do not know when ANCA becomes positive during the three phases of EGPA progression. Early intervention with anti–IL-5 in severe eosinophilic patients, as illustrated in case 3, may therefore prevent development of vasculitis. Nevertheless, this hypothesis is difficult to prove because of the rarity of this disease,” Tamaki commented. “Future data are awaited on EGPA prevention through early intervention with anti–IL-5 and its effects on severe eosinophilic cases.”
Clinical guidelines for EGPA management
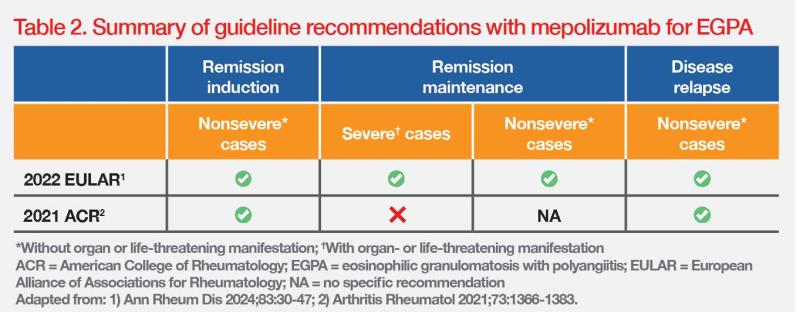
The management approach for EGPA is typically based on a patient’s clinical features and severity, which may result in different responses to treatment. For nonsevere cases (ie, without organ or life-threatening manifestation), the American College of Rheumatology (ACR) and European Alliance of Associations for Rheumatology (EULAR) recommend mepolizumab for remission induction in relapsing or refractory EGPA. After remission induction, mepolizumab is also recommend for remission maintenance, irrespective of EGPA severity, and for EGPA patients with residual glucocorticoid-dependent asthma who achieved remission of major organ involvement. (Table 2) [Arthritis Rheumatol 2021;73:1366-1383; Ann Rheum Dis 2024;83:30-47]