Two patients with advanced endometrial cancer treated with chemo-IO





Case 1: Reduction in residual tumours after two cycles of chemo-IO
History, presentation and investigations
A 59-year-old female was referred for management of suspected primary carcinoma of the peritoneum in August 2023. She complained of right flank pain and abdominal distention during the previous month. An abdominopelvic (AP) CT scan showed right hydronephrosis and hydroureter along with multiple peritoneal and omental (largest 4.3 x 12.1 x 4.5 cm) masses, as well as a pancreatic tail lesion. There were no enlarged lymph nodes (LNs). Gross ascites and moderate left pleural effusion were present without a pleural mass. Her Eastern Cooperative Oncology Group performance status (ECOG PS) was 1.
An omental mass biopsy revealed high-grade adenocarcinoma with spindle cell areas. Somatic testing for homologous recombination deficiency was negative. Immunohistochemistry (IHC) confirmed mismatch repair-proficient (pMMR) tumour type. Abdominal ascitic fluid and pleural fluid cytology was positive for metastatic carcinoma. Based on these findings, the patient was initially diagnosed with stage IV adenocarcinoma, possibly originating from the ovary or peritoneum.
Treatment and response
A regimen of bevacizumab (15 mg/kg) plus carboplatin (area under the concentration-time curve [AUC], 5 mg/mL/min) and paclitaxel (175 mg/m2) every 3 weeks was initiated on 30 August 2023.1 At the end of cycle 1 (10 days post chemotherapy), the patient was admitted with abdominal pain, fever and elevated C-reactive protein. An AP CT scan performed on 9 September 2023 confirmed large bowel perforation, likely related to bevacizumab use.2 The patient underwent exploratory surgery with right hemicolectomy and ileostomy on 13 September 2023. Pathological analysis showed the presence of sarcomatous elements, which prompted a change in her diagnosis to carcinosarcoma.
In view of the above complication, the second cycle of carboplatin and paclitaxel was deferred for 1 week and given on 28 September 2023, without bevacizumab. The patient was reluctant to add another immunotherapy (IO) agent at that time. After completion of another five cycles of chemotherapy, a PET-CT scan in January 2024 showed good partial response within peritoneal metastases, but also an enlargement of the pancreatic tail lesion.
Dostarlimab (500 mg) was added to carboplatin and paclitaxel in January 2024 to enhance response, especially in the pancreatic metastasis.3 A CT scan in February 2024 following two cycles of chemo-IO showed further reduction in previous residual tumours, including the pancreatic tail lesion.
After four cycles of chemo-IO, the patient underwent total hysterectomy and bilateral salpingo-oophorectomy (THBSO) with peritoneal nodule biopsy in April 2024, which showed a good response to treatment with very little residual disease left. Her ECOG PS remained at 1. She tolerated dostarlimab well with no skin reactions or other adverse effects other than those related to chemotherapy. She received two more cycles of dostarlimab maintenance (1,000 mg every 6 weeks) postoperatively.
Unfortunately, the patient’s CA-125 level rose to 138 U/mL and multiple peritoneal recurrences were noted in an AP CT scan performed in August 2024. As a result, IO was stopped and second-line chemotherapy with liposomal doxorubicin was initiated later that month.
Case 2: Ongoing response after four cycles of maintenance IO
History, presentation and investigations
A 61-year-old female presented with left lower quadrant pain and postmenopausal bleeding in August 2023. She was previously seen by a gynaecologist and was found to have atypical glandular cells on a Pap smear. Cervical biopsy in October 2023 showed detached fragments of adenocarcinoma, which was confirmed by endocervical and endometrial curettages done on the same day.
A pelvic MRI in November 2023 revealed an endometrial tumour measuring 6.6 x 5.5 x 7.2 cm. The cancer had invaded through the myometrium with extensive metastases in the peritoneum, involving multiple organs (including the rectum) and LNs in the pelvic area. A PET scan done on the same day also showed widespread peritoneal and LN metastases reaching diaphragmatic stations. Her disease was classified as stage IV (T3N1M1) endometrial cancer (EC).
IHC staining revealed that the tumour was pMMR and had HER2 equivocal (2+) status. Subsequent HER2 gene amplification by fluorescence in‐situ hybridization test was negative.
Treatment and response
The patient completed a total of six cycles of dostarlimab (500 mg) plus carboplatin (AUC, 5 mg/mL/min) and paclitaxel (175 mg/m2) every 3 weeks between November 2023 and January 2024.3 An AP CT scan in January 2024 after three treatment cycles and a PET-CT scan in March 2024 after six cycles showed good partial response. Exploratory surgery was performed in April 2024, with THBSO, omentectomy and peritoneal biopsy. The bulk of the original tumour in the pelvis had disappeared following treatment, and continued remission was observed at distant sites. Her ECOG PS improved from 1 before treatment to 0 after the surgery.
The patient had since received four cycles of dostarlimab maintenance (1,000 mg every 6 weeks). In addition, radiation therapy was given to treat any residual disease in the pelvis after the surgery. Dostarlimab maintenance is expected to continue for a total of 3 years, until disease progression or unacceptable toxicity. The patient tolerated dostarlimab very well, without any complications.
Discussion
The National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines currently include dostarlimab plus carboplatin and paclitaxel as one of the category 1, preferred, first-line systemic therapy options for primary advanced or recurrent EC. The US FDA recently expanded dostarlimab’s indication to include all patients with primary advanced or recurrent EC, irrespective of MMR/microsatellite instability status.3 The recommendation and approval are based on the results of the phase III RUBY trial (n=494), which demonstrated that the addition of dostarlimab to carboplatin and paclitaxel significantly increased progression-free survival (PFS) among patients with primary advanced or recurrent EC, regardless of MMR/microsatellite instability status.
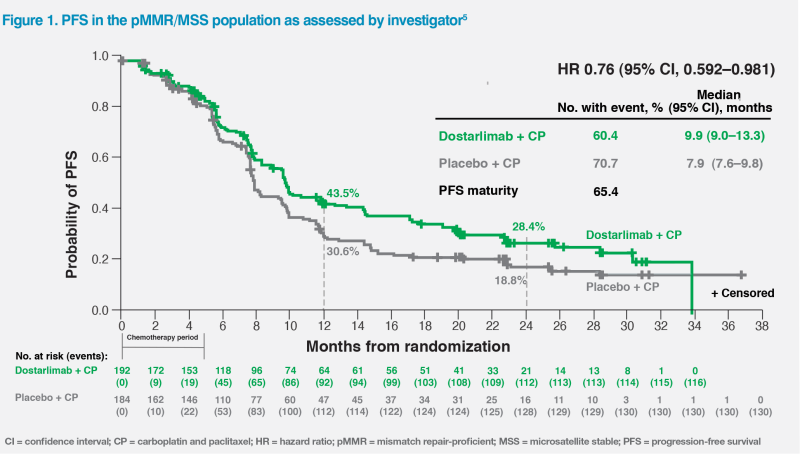
Among patients with pMMR/microsatellite-stable (MSS) tumours, such as our two patients, who represented 76.1 percent of the trial’s overall population, the 24-month PFS rate was 28.4 percent with dostarlimab vs 18.8 percent with chemotherapy alone (hazard ratio [HR], 0.76; 95 percent confidence interval [CI], 0.592–0.981).4 (Figure 1)
Furthermore, at a median follow-up of 37.2 months and 51 percent data maturity for the overall population, RUBY met its coprimary endpoint of OS, demonstrating a statistically significant reduction in the risk of death in patients treated with dostarlimab plus carboplatin and paclitaxel vs carboplatin and paclitaxel alone (HR, 0.69; 95 percent CI, 0.54–0.89; stratified log-rank p=0.0020). Median OS was 16.4 months longer in the dostarlimab vs chemotherapy alone arm (44.6 vs 28.2 months).6
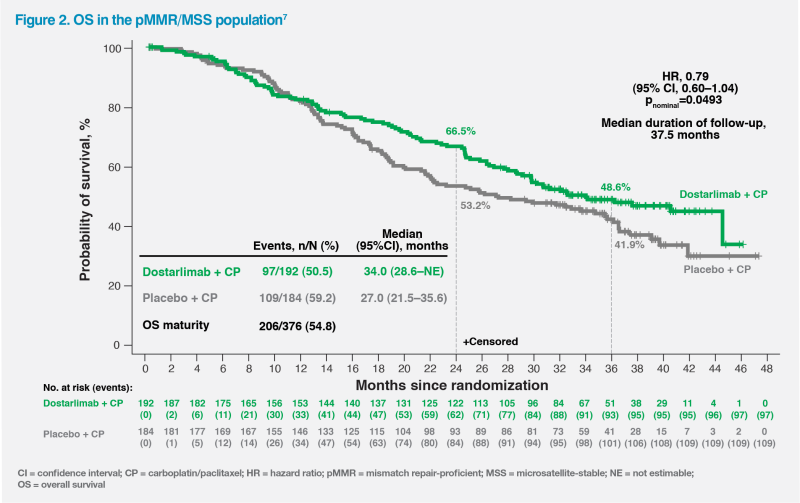
At 54.8 percent OS data maturity, a trend in favour of dostarlimab was also seen in the pMMR/MSS population, which suggested a 21 percent reduction in the risk of death (HR, 0.79; 95 percent CI, 0.60–1.04, nominal p=0.0493) – a notable finding given that 37.0 percent of pMMR/ MSS patients in the placebo arm received IO as the first subsequent anticancer therapy after dostarlimab failure. Median OS was 34.0 vs 27.0 months with vs without dostarlimab.6 (Figure 2)
At present, dostarlimab, added to carboplatin and paclitaxel, is the only agent with phase III trial data to demonstrate statistically significant OS improvement vs the previous standard-of-care carboplatin and paclitaxel alone in primary advanced or recurrent EC.
As well as demonstrating clinically meaningful improvements in survival, the RUBY trial is notable for its broad inclusion criteria, meaning that dostarlimab’s efficacy was shown in a wide population of patients. For instance, patients with rarer high-risk histologic subtypes, such as carcinosarcoma, like our first case, were eligible for the trial, making dostarlimab an appropriate choice for this patient.4
Overall, our two patients with pMMR/MSS stage IV EC achieved good partial response with dostarlimab plus chemotherapy and tolerated their treatment well. Their experience is consistent with results of the RUBY trial, which has the longest follow-up and most robust phase III survival data in advanced or recurrent EC to date.