Use of an amyloid β–directed antibody in Alzheimer’s disease









Presentation
A 72-year-old male retired university lecturer with no family history of memory issues recently exhibited signs of memory decline, as observed by his family. The patient did not perceive any significant memory issues in himself and did not view himself as forgetful. He acknowledged that he may not remember things if he lacked interest in them or could not connect them to something familiar.
The patient’s wife observed that the patient had difficulty using his cell phone and experienced decreased spelling ability. Over the past few months, she noted instances where the patient recognized places as familiar, even though they had never visited those places before. Additionally, she had to take over travel arrangements for their current trip – a task he usually handled.
The patient’s daughter noticed that when the patient was under stress, he tended to repeat questions frequently, sometimes asking the same question up to five times in an hour. The patient also showed signs of confusion and forgetfulness, such as mistaking one daughter’s dog for the other’s or repeatedly asking to see his daughter’s new apartment despite having already viewed it over video chat.
The patient believed his family might be overreacting and asked them to ascribe these observations to normal ageing. He expressed feelings of being controlled by his family and not having enough freedom. His daughter speculated that the patient’s disinterest in technology could contribute to his perceived detachment, as he did not keep up with the family group chat where plans and schedules were being discussed. Other close acquaintances also noticed changes in the patient’s behaviour, which he denied.
The patient experienced increased tiredness over the last few months. He believed he had no sleeping problems, although his wife confirmed he had sleep disturbance and often became tired around lunchtime, leading to him taking a nap. Because of that, the patient reduced some of his activities, particularly taking on less responsibility in a Bible study group.
Mental capacity was examined by the Montreal Cognitive Assessment (MoCA). The patient scored 25 out of 30, indicating impairment of memory and orientation. This score is above the 16th percentile after adjustment for age and education. MRI brain showed cerebral atrophy consistent with Alzheimer’s disease (AD), with an atrophy index (AD-RAI) of 98 percent. PET scan confirmed high Pittsburgh compound B (PIB) retention over the posterior cingulate gyrus, precuneus, temporal, and parietal lobes, indicating high amyloid deposition. This was accompanied by mild hypometabolism on FDG PET scan. Blood test for apolipoprotein E (APOE) showed heterozygous alleles (ε3/ε4).
Treatment and response
The patient was initially prescribed a bundle of supplements and nutrient support to address his declining cognitive function. Oral drugs for AD were also initiated, but the effect was suboptimal and their use was limited due to side effects. He was subsequently started on antidepressants to manage sleeping problems and irritability.
In late 2023, intravenous lecanemab 480 mg once every 2 weeks was introduced as a new treatment for AD. The patient did not experience any infusion-related reactions or other clinically detectable side effects. After the fourth dose, his wife reported an improvement in his verbal output, and he was able to participate more actively in the Bible study group. His conversations with relatives and friends also became more natural and engaging.
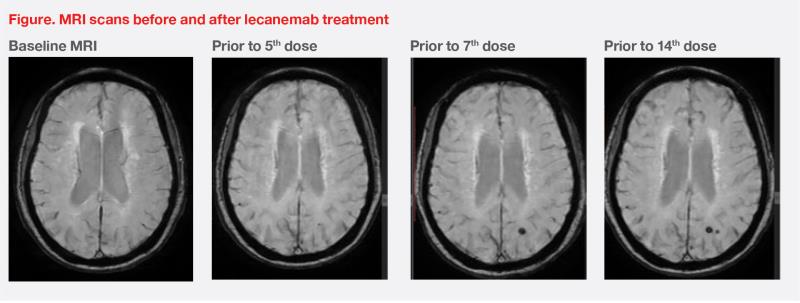
During the lecanemab treatment period, MRI showed a tiny new microbleed in the left occipital region without any accompanying perifocal oedema. The finding was classified as a mild case of amyloid-related imaging abnormality – haemosiderin deposition (ARIA-H). Prior to the 14th dose of lecanemab, another tiny microbleed was detected adjacent to the first one. Despite these findings, treatment continued without any additional symptoms. (Figure)
Discussion
The hallmark and earliest neuropathologic change that characterizes AD is amyloid pathology (A), tauopathy (T), and neurodegeneration (N); these can be incorporated into the current diagnostic framework, also known as the “A/T/N” system.1 Since the 2000s, numerous large clinical trials with the ambition of targeting amyloid were initiated; the first entity that received full US FDA approval was lecanemab (in 2023), an anti-human amyloid peptide monoclonal antibody.2-3
The efficacy of lecanemab has been investigated in its landmark trial, CLARITY-AD, in 1,795 patients with mild cognitive impairment (MCI) or mild dementia due to AD. Results showed that lecanemab significantly reduced the decline in cognitive function by 27 percent vs placebo (adjusted mean difference in Clinical Dementia Rating – Sum of Boxes [CDR-SB] score, 0.45; 95 percent confidence interval [CI], -0.67 to -0.23; p<0.001) in an 18-month study period.4
All secondary endpoints were met. In particular, lecanemab reduced amyloid burden on PET scan vs placebo (adjusted mean difference, 59.12 centiloids; 95 percent CI, -62.64 to -55.60; p<0.001). After 18 months of treatment, 68 percent of lecanemab-treated patients were considered amyloid-negative (ie, <30 centiloids).4-5
The CLARITY-AD study included an optional tau PET substudy (n=342), where patients were stratified into low tau (defined as whole cortical grey matter tau standardized uptake value ratio [SUVr] <1.06; n=141), intermediate tau (SUVr, 1.06–2.91; n=191), and high tau (SUVr, >2.91; n=10) populations. Notably, in the low tau population, more patients treated with lecanemab vs placebo demonstrated no decline (76 vs 55 percent; p=0.024) or an improvement (60 vs 28 percent; p=0.0007) in CDR-SB scores at 18 months.6
Lecanemab not only removed amyloid, but also significantly slowed tau spread in different brain regions, including the medial temporal (adjusted difference, -0.068; p=0.0024), meta temporal (adjusted difference, -0.071; p=0.012), and temporal (adjusted difference, -0.065; p=0.016) lobes.6
The most common adverse events (AEs) in the lecanemab group were infusion-related reactions (any grade, 24.7 percent; grade 1–2, 96.5 percent) and ARIA, including oedema/sulcal effusion (ARIA-E) and ARIA-H (eg, microhaemorrhages [mH], superficial siderosis). The overall rates of ARIA-E (any grade, 13 vs 2 percent) and ARIA-H (any grade, 17 vs 9 percent) were elevated with lecanemab vs placebo, but isolated ARIA-H did not appear to be related to lecanemab (9 vs 8 percent).7 Of note, ARIA may occur spontaneously in AD or because of treatment.
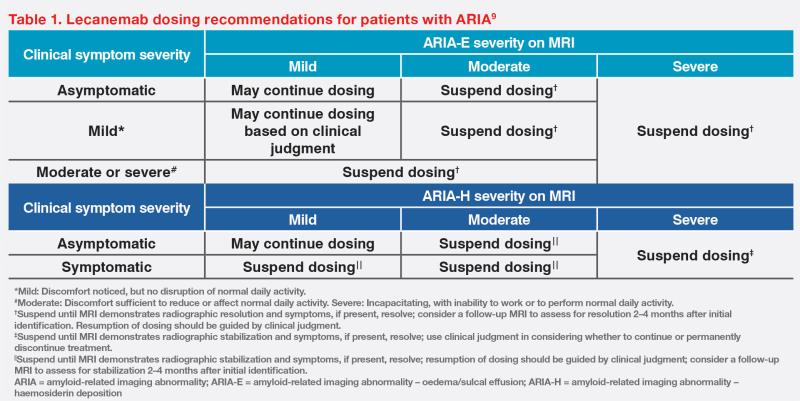
The majority of ARIA events are asymptomatic or may have nonspecific presentation.8 In CLARITY-AD, symptomatic ARIA only occurred in a small proportion of patients (ARIA-E, 3.3 percent; ARIA-H, 1.7 percent).7 As shown in our case, lecanemab treatment can be continued despite radiographically mild or asymptomatic ARIA. However, symptomatic and radiographically moderate-to-severe ARIA may warrant treatment suspension.9 (Table 1) Importantly, individual analyses suggested that ARIA events did not impact patients’ cognition, function, and treatment efficacy.7
APOEε4 status may be informative for risk stratification strategy. ARIA-E was more common in APOEε4 carriers (homozygotes, 34.5 percent; heterozygotes, 11.6 percent) vs noncarriers (6.5 percent). ARIA-E generally occurred within the first 3 months (71 percent) or 6 months (92 percent), tended to be radiographically mild-to-moderate (88.5 percent) and asymptomatic (96.7 percent), and resolved within 4 months of detection (81 percent), regardless of APOEε4 status.7
Concurrent ARIA-H and ARIA-E events were likely to occur early in the course of treatment. APOEε4 carrier status contributed to ARIA-H incidence but not timing. ARIA-H events were mostly radiographically mild to moderate (80.5 percent) and asymptomatic (90.9 percent). In most patients (83 percent) with radiographically mild ARIA-H, this did not deteriorate and treatment could be continued at the same dose without interruption. Of note, 80 percent of cases of first mild radiographic ARIA-H were stable at the next MRI scan.7
Scheduled MRI scans were taken for our patient at baseline and before the fifth, seventh, and 14th infusions for ARIA monitoring. Table 2 describes the minimal and recommended MRI imaging sequences for scheduled scans.10 For symptomatic patients and differential diagnosis (eg, stroke, infection, and posterior reversible encephalopathy syndrome), structural T2 and pre- and post-contrast T1 images should be added to the usual sequences.