
History and presentation
A 66-year-old man with plasma cell myeloma and high-risk cytogenetic abnormality was referred to our centre in September 2022. He previously visited a private clinic in August 2022 for back and rib pain, where he received his earlier diagnosis. He was a smoker and chronic drinker with a history of hypertension. His Eastern Cooperative Oncology Group performance status (ECOG PS) was 0–1.
Initial investigation before referral revealed multiple lytic lesions in the skull and pelvis. Blood tests showed mild anaemia (haemoglobulin [Hb], 10.2 g/L), normal calcium level and slightly elevated creatinine level (112 μmol/L). Creatinine clearance was 59 mL/min, and an inversed ratio of albumin to globulin was found. Bone marrow examination revealed up to 98 percent plasma cells, and fluorescence in situ hybridization (FISH) detected translocation t(4;14).1
Immunofixation carried out at our hospital showed an elevated level of immunoglobulin G (IgG; 72.23 g/L), with IgG kappa paraprotein of 51 g/L in serum protein electrophoresis. Serum free light chain (FLC) assay demonstrated a kappa/lambda FLC ratio of 6.4 (kappa FLCs, 18.1 mg/L; lambda FLCs, 2.8 mg/L). PET-CT in December 2022 revealed multiple lytic lesions in the axial and appendicular skeleton, with no extramedullary lesions.
These findings were consistent with International Staging System (ISS) stage II, Revised-ISS stage II, high-risk, transplant-ineligible newly diagnosed multiple myeloma (TIE-NDMM).1
Treatment and response
In late September 2022, the patient initiated DRd regimen of intravenous (IV) daratumumab (16 mg/kg weekly for 8 weeks, then every 2 weeks up to week 24, and monthly thereafter) combined with lenalidomide (uptitrated from 10 mg to 15 mg on days 1–21) and dexamethasone (20 mg twice weekly). IV methylprednisolone, IV chlorphenamine, and oral paracetamol were given before each infusion to avoid infusion-related reaction (IRR). On the basis of pretreatment screening, entecavir was administered to prevent hepatitis B virus (HBV) reactivation, and cotrimoxazole and aspirin were given for prophylaxis of pneumocystis pneumonia (PCP) and thrombosis, respectively.
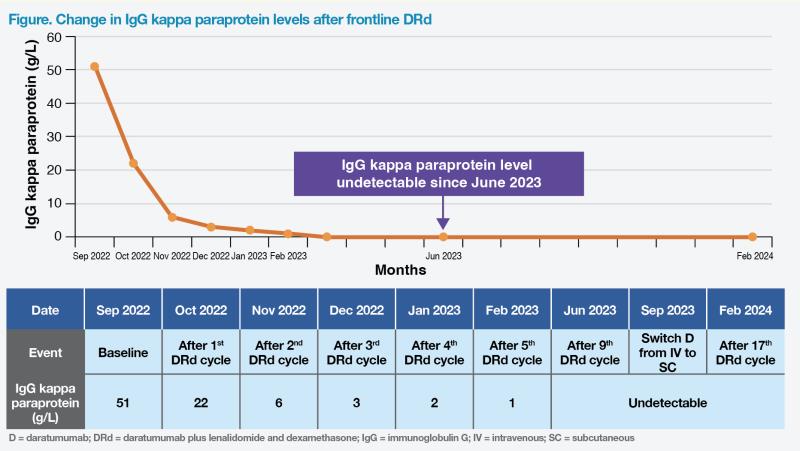
After one cycle of DRd, the patient experienced decreased bone pain, accompanied by normalization of Hb level. After two cycles, his bone pain completely resolved. Notably, his IgG kappa paraprotein level decreased from 51 g/L to 22 g/L after one cycle, and gradually declined to an undetectable level in June 2023. These findings suggested that the patient met complete response (CR), although bone marrow aspiration was not conducted.1 (Figure)
During this period, the patient experienced grade 2 neutropenia, and lenalidomide dose remained at 15 mg without further uptitrating to 25 mg.2 No granulocyte colony–stimulating factor (G-CSF) support was required.
In September 2023, route of daratumumab administration was switched from IV to subcutaneous (SC). During a visit in late February 2024, the patient remained asymptomatic despite grade 2 neutropenia, with an ECOG PS of 0. His Hb level was 15 g/L, and IgG paraprotein remained undetectable. (Figure) He continued to receive DRd, with the 19th and 20th cycles scheduled.
Discussion
According to clinical consensus presented at European Hematology Association 2023 Congress, using effective frontline treatments is important for TIE-NDMM patients.3 Introducing potent agents (eg, anti-CD38 monoclonal antibody [mAb]) to frontline treatment may enhance clinical outcomes.4
Our patient, initially diagnosed with MM at the age of 66 years, was deemed unfit for transplantation.5 Considering his high-risk disease status and absence of financial concerns, he chose the anti-CD38 mAb–based DRd regimen, which is one of the preferred regimens (category 1) for TIE-NDMM patients in guidelines of National Comprehensive Cancer Network (NCCN).1
NCCN’s recommendation of DRd for TIE-NDMM patients is based on results of the phase III MAIA trial (n=737).1,2 In the prespecified interim analysis at a median follow-up of 28.0 months, the primary endpoint of progression-free survival (PFS) was significantly improved with DRd vs lenalidomide plus dexamethasone (Rd) (hazard ratio [HR], 0.56; 95 percent confidence interval [CI], 0.43–0.73; p<0.001).2
In updated analysis of MAIA at a median follow-up of 64.5 months, DRd significantly reduced the risk of disease progression or death by 45 percent vs Rd (median PFS, 61.9 vs 34.4 months; HR, 0.55; 95 percent CI, 0.45–0.67; p<0.0001).6 DRd-associated PFS improvement was consistent across most subgroups, including patients with high cytogenetic risk (HR, 0.57; 95 percent CI, 0.34–0.96) and those with revised high cytogenetic risk, such as gain(1q21) (HR, 0.43; 95 percent CI, 0.24–0.76).7
At a median follow-up of 73.6 months, overall survival was significantly improved with DRd vs Rd (median, not reached vs 64.1 months; HR, 0.65; 95 percent CI, 0.52–0.80; p<0.0001).6
The most common haematologic adverse event (AE) in the DRd group was neutropenia (any grade, 61.5 percent; grade 3/4, 54.1 percent). Other grade 3/4 AEs included infection (42.6 percent) and pneumonia (19.5 percent).6 While pneumonia is rare in our practice, PCP prophylaxis can be considered, which was implemented for our patient. G-CSF support may be required in severe neutropenia cases.
In MAIA, IRRs occurred in 40.9 percent of patients in the DRd group, of whom 98 percent experienced IRR during first exposure to daratumumab. Only 2.7 percent of IRRs were classified as grade 3/4 events.2 To reduce IRR risk, patients should be premedicated with methylprednisolone, antipyretics, and antihistamines. Patients receiving daratumumab monotherapy require postmedication with prednisolone on days 2 and 3 following daratumumab administration, which may be omitted in combination therapy.8
HBV screening should be performed before daratumumab treatment initiation, with positive cases requiring HBV prophylaxis. Given frequent needs for blood transfusion in MM management, red blood cell phenotyping/genotyping may be part of pretreatment screening.1,8
Our patient had complete resolution of bone pain after only 2 months of DRd treatment and remained progression-free at his latest assessment in February 2024, which translates to an ongoing PFS of ≥1.5 years. Despite grade 2 neutropenia requiring a reduced lenalidomide dose, he had stable condition and continued DRd treatment. Like most of our patients, he transitioned from IV to SC daratumumab, which is widely adopted in public hospitals in Hong Kong because of its wide availability, equivalent efficacy to IV formulation and shorter clinic time.9
In summary, DRd is an effective and well-tolerated frontline regimen for TIE-NDMM patients, as demonstrated by our high-risk patient case.