Use of maintenance immunotherapy as bladder-preserving therapy in node-positive muscle-invasive urothelial carcinoma





Presentation and initial treatment
A 45-year-old nonsmoking, non-drinking Chinese male with good past health presented in 2019 with haematuria. He was initially diagnosed with non–muscle invasive urothelial carcinoma (UC) of the bladder. Transurethral resection of bladder tumour (TURBT) was performed in June and August 2019, followed by three courses of intravesical Bacillus Calmette-Guerin between September 2019 and May 2020. His baseline Eastern Cooperative Oncology Group Performance Status (ECOG PS) was 1.
Investigation and treatment for MIBC
Follow-up cystoscopy in July 2021 showed nodules in the bladder neck, necessitating repeat TURBT. Nodule biopsy and pathological examination revealed high-grade muscle-invasive bladder cancer (MIBC). PET-CT scan showed a 4.6 cm bladder base tumour extending into the bladder neck and prostatic urethra with bilateral pelvic lymph node (LN) involvement. (Figures 1A and 1B) There was no evidence of distant metastases. Findings were consistent with stage III, T4N2M0 MIBC.
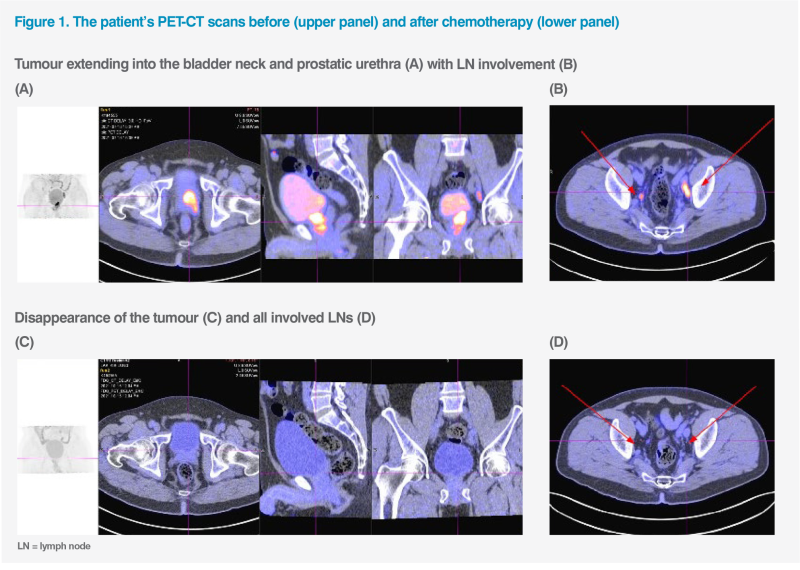
As the patient refused consolidative radical cystectomy, he received four cycles of chemotherapy with gemcitabine (1,250 mg/m² on days 1 and 8) and cisplatin (75 mg/m² on day 1, Q3W) from July to October 2021. Postchemotherapy PET-CT scans showed complete response in the tumour and LNs. (Figures 1C and 1D) Concurrent chemoradiotherapy (cCRT; 55Gy in 20 fractions) with weekly cisplatin 40 mg/m2 was completed in November 2021.
In January 2022, the patient started maintenance therapy with intravenous avelumab 10 mg/kg Q2W to maintain disease control and delay progression. Cystoscopy in August 2023 confirmed ongoing complete remission. The latest PET-CT scan in March 2024 showed no recurrence or distant metastasis.
The patient experienced self-limiting grade 1 skin itchiness and fatigue during avelumab treatment, which did not impact his daily activities. No immune-related adverse events (irAEs) were observed, and his quality of life (QoL) was maintained.
Last seen in late August 2024, the patient was asymptomatic, had an ECOG PS of 0, and was able to work normally. He remained in complete remission for >2.5 years with avelumab maintenance therapy.
Discussion
Although our patient with T4N2M0 MIBC had no distant metastasis, the extensive local invasion rendered him inoperable. Therefore, a treatment approach for advanced or metastatic UC was taken.
According to the European Society for Medical Oncology (ESMO) guideline, cisplatin- or carboplatin-based chemotherapy (both I, A) followed by maintenance avelumab (I, A) in those who remain progression-free following chemotherapy is one of the first-line treatment strategies for advanced or metastatic UC. This approach applies to both cisplatin-eligible and cisplatin-ineligible patients, including ours.1
The EV-302 trial evaluating the efficacy of enfortumab vedotin (EV) and pembrolizumab showed promising results in previously untreated advanced UC.2 However, some patients may be ineligible for EV treatment if they meet ≥2 of the following EV-ineligible criteria (EVITA):3
First-line avelumab maintenance is a recommended first-line treatment for both cisplatin-eligible and cisplatin-ineligible patients with advanced or metastatic UC based on findings from the phase III JAVELIN Bladder 100 trial. Overall, 700 patients were randomized 1:1 to receive avelumab plus best supportive care (BSC) or BSC alone.4,5
After ≥2 years of follow-up (median, 38.0 months for avelumab, 39.6 months for controls), 19.5 percent of patients received avelumab treatment for ≥2 years. Avelumab maintenance continued to show significantly prolonged investigator-assessed progression-free survival (PFS; median, 5.5 vs 2.1 months; hazard ratio [HR], 0.54; 95 percent confidence interval [CI], 0.46–0.64; 2-sided p<0.0001) and overall survival (OS; median, 23.8 vs 15.0 months; HR, 0.76; 95 percent CI, 0.63–0.91; 2-sided p=0.0036) vs control. Rates of investigator-assessed confirmed objective response (14.3 vs 4.0 percent) and disease control (50.9 vs 34.0 percent) were higher with avelumab vs control.6 Similarly, our patient achieved durable local and systematic control with avelumab maintenance, with PFS ≥2.5 years.
Of note, our patient’s ECOG PS improved from 1 to 0 and he was able to continue working daily, indicating that the addition of avelumab maintenance either improved or at least had no detrimental effect on his QoL. His experience reflects patient-reported outcomes in JAVELIN Bladder 100, which showed comparable time to deterioration in disease-related symptoms (NCCN/FACT Bladder Symptom Index-18 total score, 52.51 vs 52.41) and QoL measures (European Quality of Life 5 Dimensions 5 Level Version [EQ- 5D-5L] index score, 0.77 vs 0.75; EuroQol visual analogue scale [EQ-VAS], 76.45 vs 75.39) with avelumab vs control.7
Longer-term safety data of JAVELIN Bladder 100 were consistent with previous analyses, showing no new safety signals.6 Although our patient did not experience irAEs, these can affect any organ and system and often have a delayed onset. Therefore, it is essential to remain vigilant for signs of irAEs, such as dyspnoea, diarrhoea, and jaundice following treatment with avelumab.8
As illustrated in our patient with node-positive, nonmetastatic MIBC, avelumab maintenance is integral to multimodal bladder-preserving treatment, which consists of maximal TURBT, upfront chemotherapy, and concurrent cCRT for local and systemic control, followed by avelumab maintenance therapy. Optimal candidates for the bladder-preserving treatment approach are patients with smaller solitary tumours, absence of extensive or multifocal urinoma in situ, no tumour-related hydronephrosis, and adequate pretreatment bladder function.9
The choice of second-line treatment depends on patients’ prior treatments and biomarkers. As the treatment landscape evolves, options may include antibody-drug conjugates (eg, EV, trastuzumab deruxtecan for HER-2 expressing tumours), immune checkpoint inhibitors, FGFR-targeted therapy, and chemotherapy.1,10